G. Nagaraja Perumal , V. E. Idachristi, M. Karpakavalli and S. Mohan
, V. E. Idachristi, M. Karpakavalli and S. Mohan
Karpagam College of Pharmacy, Coimbatore, Tamil Nadu, India.
Corresponding Author E-mail: tgnp.1979@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1581
Abstract
Diabetes mellitus (DM) is most significant health problem in various developed and developing countries due to alteration of various clinical and pathological factors. Current work was intended for examine in vitro and in vivo anti diabetic competence of Cassia Auriculata flowers and its phytochemical analysis. The inhibitory effects on carbohydrate digestive enzyme α- amylase interaction with various extracts of Cassia Auriculata were contrast with acarbose. Hypoglycemic activity was executed with standard as a glibenclamide. Study indicated ethanolic extract showed higher action on α- amylase inhibition with assessment of IC50 value; 43.6%. Based on the above results of in vitro studies were used to selected ethanolic extract of Cassia Auriculata flower used for further study. Results of animal studies indicated that the ethanolic extract of C. Auriculata has shown dose dependent action (200 mg/ kg (1.20±0.91↓) and 400 mg / kg (4 ± 0.01↓) when compared to control and standard drug treated groups. Our study confirmed to ethanolic extract work through the α- amylase inhibition mechanism. Our view bioactive constituents confirm anti diabetic capacity and afford methodical source for validation of Cassia Auriculata flowers in ayurvedic formulations on diminution of DM prevalence.
Keywords
Acarbose; Alfa Amylase and Hypoglycemic; Cassia Auriculata; Glibenclamide
Download this article as:| Copy the following to cite this article: Nagarajaperumal G, Idachristi V. E, Karpakavalli M, Mohan S. In Vitro and In Vivo Evaluation of Potential Anti Diabetic Efficacy on Cassia Auriculata Flowers. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Nagarajaperumal G, Idachristi V. E, Karpakavalli M, Mohan S. In Vitro and In Vivo Evaluation of Potential Anti Diabetic Efficacy on Cassia Auriculata Flowers. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24274 |
Introduction
Free radicals were extremely hasty chemical species frequently produced in the human system by usual organic reactions on various exogenous systems.1 A number of radicals were involved in the process of biological functions. Consequently, their effects on the organism are plaid by protection system that includes various enzymes which are excoriated oxidative stress and dent cells. Amplified oxidative stress directed diabetes mellitus and complications. Accordingly, endorsement on curative natural agents requires methodical exploration evaluation of effectiveness with variety of Ex vivo systems, besides properties involving whole animal preparation.2
Identified plant has with splendid alluring brilliant golden yellow blossoms yellow dispersed in dry parts of India and Asia. A dissimilar fraction of plant has been reported on the survey articulate practice of plant against constipation, skin disorder and various other disorders. In addition, flora used to prepared various formulations counteractive effect on DM.3 Dissimilar extracts ready by use flowers expressed an assortment of actions.4,5 Traditional medicine flower part of Cassia Auriculata incited investigated in features, avert oxidation efficiency, anti diabetic action, plummeting clout, shifting ferrous ions, anti-peroxidation and ROS amend capabilities were in vitro conditions. Survey report of C. Auriculata has been illustrated assorted limitation were determined whole animal preparation but no proper scientific validation. In current research required to be finding as a plant based new entity for the treatment against diabetes mellitus and also perform complete scientific validation of above plant.6,7
Materials and Methods
Identification and Authentication
Cassia Auriculata blossoms were collected at Coimbatore District. Plant matters recognized and genuinely checkered at Botanical survey of India, Coimbatore No: BSI / SRC / 5 / 23 / 2012-13 Tech / 496.
Preparation of Extract
The blossoms were isolated, washed altogether with water and shade dried for 6 days. 1000 grams of powdered blossoms was subjected to extraction with methanol, ethanol, chloroform, petroleum ether, ethyl acetate (2000ml) in a round bottom flask at room temperature for 15days.8 After fifteen days decanted and press the mark up to collect the fluidized product which were resolute utilizing rotating vacuum evaporator under lessened weight for collected extract 17.8%.
Phytochemical Test
Screening and identification of phytochemical constituents observational study was done in standard methodology.
Mayer’s Reagent
Required quantity of mercuric chloride + 60 ml of refine water + 5.0 g of potassium iodide were mix 20 ml of refine water water. Both solutions were mixed and volume was raised to 100 ml with distilled water.9
Dragendorff’s Reagent
First preparation: 1.7 grams of basic bismuth nitrate and 20 g of tartaric acid added 80 ml of refine water in a 100 ml standard flask. Second preparation: 16 grams of potassium iodide + 40 ml of refine water. First + Second mixed equal ratio.9
Test for Alkaloids
About 0.6 g of plant extract + 8 milliliter of 1% Hydrochloric acid temperate and filtered. Required quantity of filtrate mixed both reagents such as Mayer’s and Dragendorff‘s.
Test for Steroids
0.7g gram of plant extract was diverse with two milliliter of acetic anhydride chase by two milliliter of sulphuric acid.9
Test for Terpenoids
Required quantity of plant extract was adding two milliliter of chloroform in a test tube and added three milliliter of concentrated sulphuric acid.8
Test for Flavonoids
Substance treated alcohol, a couple of magnesium turnings and few drops of concentrated HCL were added and bubbled for five minutes.9
Test for Tannins
0.5 gram of sample boiled in twenty milliliter of refine water in test tube and filtered.9
Test for Phytosterol
Sample was liquefying in Two milliliter of acetic anhydride, animated for sweltering, cooled and one milliliter of concentrated sulfuric acid was added.9
Foam Test: 5 milliliter test solution taken single test tube was disturbed well for 5 mins.
Olive oil test: – Additional a couple of olive oil to required quantity in test tube congaing sample.9
Test for Glycosides
Keller -Killiani test: Additional required quantity of glacial acetic acid + few drops of 5 % ferric chloride solution to a little of dry extract. Further 0.5 ml of concentrated sulfuric acid was added along the side of the test tube carefully.9
In vitro α- Amylase Inhibition Activity 10-11
Five hundred micro liter of samples in a test tube and additional to Five hundred micro liter of 0.20 mM buffer of phosphate containing α-amylase solution and incubate at 25°C for 10 minutes. Five hundred micro liter of one percentage starch solution in 0.02 M sodium phosphate buffer additional each tube. Reaction combination was incubate at 25°C for 10 minutes and mix with 3, 5 dinitro salicylic acid colour reagent which incubate boiling water bath for five minutes and cooled to room temperature then make up 10 ml refine water which absorbance estimated at 540 nm.
Proportion inhibition in each examine was intended formulae.

Experimental animals
Mice for Acute Toxicity Study
The Adult female Swiss mice weighing between (20-30 grams) were used to calculate LD50. Housed animals in clean cages and kept up under standard states of light (12 hours amid elective day/night cycles), relative humidity (60-70%) and temperature (26 ± 1°C). Experimental groups of mice was treated orally with aqueous ethanolic extract of Cassia Auriculata leaves at dose of 2000 mg/kg and observed for 15 days to register possible mortality.12-15
Preliminary In Vivo Activity
The Adult rats weight were measured range between 200-230 grams and used to perform the hypoglycemic action and Institutional Animal Ethical Committee (1164/ac/08/CPCSEA).16-21
Group 1: Control group.
Group 2: Glibenclamide 200µg/kg.
Group 3: C. Auriculata flower extract 200mg/kg.
Group 4: C. Auriculata flower extract 400mg/kg.
Animals were fasted for 48 hours and weigh the animals. Divided animals above mentioned format. Collected first drop of blood by tail nipping method and glucose level monitored by using Glucometer, which considered as a 0 hour reading. All the extracts and drug administer orally by using oral feeding needle. After the administration glucose level were constantly measured different time intervals ½ and 1 hours.
Statistical Analysis
Results were articulated as mean± SD assesses by one way analysis of variance pursued by Dunnett’s technique of several assessment.
Results and Discussion
Appearance and Percentage Yield of Extract
AEECA (Aqueous Ethanolic Extract of Cassia Auriculata ) were a semisolid brownish color extract and the percentage yield was found to be 17.8%.
Phyochemical Analysis
The phytochemical screening results revealed that the alkaloids were present due to turbidity formation. Changed from violet to blue was showed steroids. Reddish russet was formed and positive result for presence of terpenoid. Red color observed and present flavonoids. A colour change was observed in the test tube, which point out tannins present. A darker ring was development at the intersection and the turning of the upper layer to dim green which indicated the test for phytosterols present. Below two observations indicated presence of saponins due to formation of stable foam confirmed the test and formation emulsion. Formation of blue and red color. Above two color changes indicated presence of glycosides.
Table 1: Phyochemical Analysis.
| Constituents | Deduction |
| Alkaloids | + |
| Steroids | + |
| Terpenoids | + |
| Flavonoids | + |
| Tannins | + |
| Phytosterol | + |
| Saponin | + |
| Glycosides | + |
+ = Presence
Acute Toxicity
Plant a dose of 2000 mg/kg has rejection unfavorable consequences tested with mice up to 15 days of observation. Not toxic signs were absent in the mice. There was no mortality observed and recorded weight loss normal. Based on the above observation fix the doses 200 and 400 mg/kg for anti diabetic activity.
In Vitro Anti Diabetic Study
In vitro results revealed that the Alfa amylase percentage of inhibition 33.5% for Petroleum Ether extract of C. auriculata Flower by the indication pet ether extract have lesser activity when compared to ethyl acetate extract (40.2%), ethanolic extract(43.6%) of C. auriculata leaves and Acarbose (45%). In our comparative results stated that the ethanolic extracts have more alfa amylase inhibition property when compared to other extracts but acarbose have constantly higher activity when compared to extracts. Based on the above results various extracts of C. auriculata Flower working mechanism expressed given below:
Squalor of starch and complex glucose to single glucose by Alfa -amylase and Alfa-glucosidase enzymes if suppressed by block glucose absorption. Ultimately, the eminent postprandial blood sugar controlled. Numerous drugs were used to control DM and induction of stress, many are establish to adverse drug reactions. Based on the higher alfa amylase activities of Ethanolic Extract of C. Auriculata have been used for In Vivo studies.
Table 2: α-Amylase Inhibition of Petroleum Ether extract of C. Auriculata leaves.
| Concentration(µg/ml) | Percentage Inhibition (%) |
| 0 | 0 |
| 25 | 28 |
| 50 | 33.5 |
| 75 | 42.3 |
| 100 | 53.1 |
| 125 | 56.1 |
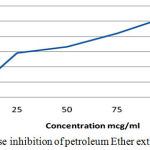 |
Figure 1: α amylase inhibition of petroleum Ether extract of C. Auriculata.
|
Table 3: α-Amylase Inhibition of Ethyl acetate Extract of C. Auriculata leaves.
| Concentration(µg/ml) | Percentage Inhibition(%) |
| 0 | 0 |
| 25 | 35.1 |
| 50 | 40.2 |
| 75 | 48.3 |
| 100 | 56.2 |
| 125 | 61 |
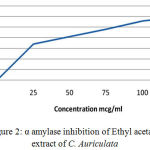 |
Figure 2: α amylase inhibition of Ethyl acetate extract of C. Auriculata.
|
Table 4: α-Amylase Inhibition of Ethanolic Extract of C. Auriculata leaves.
| Concentration(µg/ml) | Percentage Inhibition (%) |
| 0 | 0 |
| 25 | 33.6 |
| 50 | 43.6 |
| 75 | 51 |
| 100 | 55 |
| 125 | 62 |
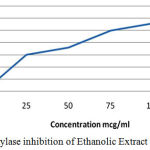 |
Figure 3: α amylase inhibition of Ethanolic Extract of C. Auriculata.
|
Table 5: α-Amylase Inhibition of Acarbose (Positive control).
| Concentration(µg/ml) | Percentage Inhibition(%) |
| 0 | 0 |
| 25 | 25 |
| 50 | 45 |
| 75 | 53 |
| 100 | 56 |
| 125 | 65 |
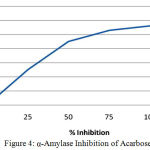 |
Figure 4: α-Amylase Inhibition of Acarbose.
|
TLC study
Percentage Inhibition of Petrolium ether extract of C. auriculata leaves = 91 µg/ml
Percentage Inhibition of Ethyl acetate extract of C. auriculata leaves = 81 µg/ml
Percentage Inhibition of Ethanolic extract of C. auriculata leaves = 74 µg/ml
Percentage Inhibition of Acarbose (Positive control) = 62 µg/ml
Minimum Percentage Inhibition was found in ethanolic extract of C. auriculata leaves which resemblance to Percentage Inhibition of positive control, So Ethanolic extract of C. auriculata contain active constituents of anti diabetic.
Rf Value range high-Polar substances present.
Rf value range low-Low polar substance present.
Solubility of compounds depend upon the polarity of solvents.
Obtained Rf values were confirmed by standard Rf values.
Rf values obtained for my extract ranges from 0.56 to 0.88, So my extract may contain compounds like flavanoids, glycosides and alkaloids. Quantity determination of total phenolics carried out with respect standard curve of gallic acid (r2= 0.99) showed 115.8 mg of extract and also quercetin standard curve (r2= 0.994) helps concentration of flavonoids observed 114.2 mg of extract.
Table 6: Results of Ethanolic extract of C. Auriculata.
| S. No. | Solvents | Concentration | Rf value |
| 1. | Toluene + Ethyl acetate | 7:3 | 0.61 |
| 2. | Toluene + Ethyl acetate + Glacial acetic acid | 5:5:1 | 0.75 |
| 3. | Petrolium ether+Chloroform | 7:3 | 0.55 |
| 4. | Ethyl acetate + Methanol | 1:1 | 0.89 |
| 5. | Hexane+Dichloro methane | 1:1 | 0.68 |
| 6. | Ethyl acetate + Methanol | 3:1 | 0.60 |
| 7. | Dichloro methane +Hexane | 3:1 | 0.73 |
Hypoglycemic Test
In clinically oral hypoglymic drugs are evaluated by using above preclinical method which help to find the hypoglycemic property for the development of new chemical entity present in the ethanolic extract of C. Auriculata The hypoglycemic study demonstrated that the ethanolic extract of C. Auriculata flowers two dose levels of glucose level milder changes were expressed in the form of reduction points of glucose level of Ethanolic Extracts of C. Auriculata 200 mg/ kg (1.20±0.91↓) and 400 mg / kg (4 ± 0.01↓) at 0.5 hrs when compared to standard drug. One hour later Ethanolic Extracts of C. Auriculata 200 mg / kg (0.35 ± 0.72↓), 400 mg / kg (1.1 ± 0.16↓) compared to Glibenclamide 200 µg / kg (10 ± 1.02↓). Based on the above results indicated that the ethanolic extract of C. Auriculata flower is working mechanism similar to acarbose.
Table 7: Hypoglycemic Test.
| Treatment | Dose
mg/kg |
Blood Glucose Level (mg/dl) | ||
| 0 min | 0.5hr | 1 hr | ||
| Control Carboxymethyl Cellulose | 0.5 % | 67.22±0.15 | 69.05±0.46 | 70.93±1.87 |
| Positive Control
(Glibenclamide) |
0.2 | 68.93±0.54 | 53.75±1.06*** | 43.04±1.2*** |
| Ethanolic Extract of C. Auriculata | 200 | 68.45±0.76 | 67.65±1.67 | 67.95±0.95 |
| Aqueous Ethanolic Extract of C. Auriculata | 400 | 68.64±0.74 | 64.65±0.42* | 65.66±0.58* |
The blood glucose levels were expressed mean ± standard error and (n= each group consist of 6 animals)(p<0.05)*, (p<0.001)**& (p<0.0001)*** as compared to each other groups.
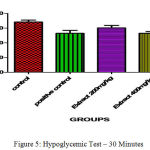 |
Figure 5: Hypoglycemic Test – 30 Minutes.
|
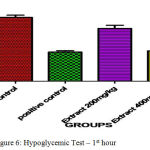 |
Figure 6: Hypoglycemic Test – 1st hour.
|
Conclusion
The results of the present study provides scientific evidence for anti diabetic activity of flowers by the evaluation of various in vitro and in vivo models and hence supports the therapeutic usage of flowers in traditional medicines for treating DM and its associated complications. This work will be useful for diabetic research workers to be found the new chemical entity for the treatment of DM and its associated diseases.
Acknowledgements
The authors are grateful to Dr. Vasanthakumar, Chairman, Karpagam Chairty Trust he valuable help rendered during the study.
References
- Andrade-Cetto A.,Becerra-Jimenez J and Cardenas-Vazquez R. Glucosidase inhibitory activity of some Mexican plants used in the treatment of type 2-diabetes. J Ethnopharmacol. 2010;116:27-32.
CrossRef - Rohan B.,and Bayer P . Enzymes of starch degradation and synthesis. Adv Enzymol. 2010;12:379.
- Hell C., Bray H. C and Thorpe W. Analysis of phenolic compounds of interest in metabolism. Meth Biochem Anal. 2015;1:27-52.
- Chang C., Yang M., Wen H and Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2010;10:178-182.
- Cotelle A., Bernier J. L., Catteau J. P., Pommery J.,Wallet J. C and Gaydou E. M. Antioxidant properties of hydroxyl flavones. Fre Radi Biol and Medi. 2012;20:35-43.
CrossRef - Divya B. T and Mini S. In vitro radical scavenging activity in different extracts of Buta monosperma bar. Int J Curr Pharm Res. 2011;3:114-11.
- Divya S., Krishna N. K.,Ramachandran S and Dhanaraju M. D.Wound healing and in vitro antioxidant activities of Croton bonplandianum leaf extract in Rats. Glob J Pharmacol. 2011;5: 159-163.
- Doshi G. M., Shahare M. D., Aggarwal G. V., Pillai P. G and Desai S. K. Evaluation of in vitro antioxidant methods of Cassia auriculata. Der Pharmacia Lettre. 2011;3:297-305.
- eward E., Elmastas M., Gulcin I., Isildak O., Kufreivioglu O. I., Ibaoglu K and Aboul-Enein H. Y. Radical scavenging activity and antioxidant capacity of bay leaf extracts. J Iran Chem Soci. 2012;3: 258-266.
- Gong., Gin H and Rigalleau V. Post-prandial hyperglycemia and diabetes. Diabetes and Metabolism. 2014;26:265-272.
- godwin G., Green L. C.,Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S and Tannenbaum S. R. Analysis of nitrate, nitrite and (15N) nitrate in biological fluid. Anal Biochem. 2015;26: 131-138.
- Gulcin I., Alici H. A and Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem and Pharmaceu Bull. 2015;53:281-285.
CrossRef - Inas S. G., Ekram S. A., Hoda F. B., Ibrahim M. F and Somaia A. N. Evaluation of antihyperglycemic action of Oyster Mushroom (Pleurotus ostreatus) and its effect on DNA damage chromosome aberrations and sperm abnormalities in streptozotocin-induced diabetic rats. J Ethanopharmacol. 2011;7:532-544.
- Joshi S. G. Cesalpinaceae-Cassia auriculata Textbook of Medicinal Plants. Oxford: IBH. 2000;4:119. Publishing. 2000.
- Kunchandy E and Rao M. N. A. Oxygen radical scavenging activity of curcumin. Int J Pharmacol. 2010;58:237-24.
CrossRef - Manogaran S and Sulochana N. Anti inflammatory activity of C. auriculata. Ancie Scie life. 2014;24:1-3.
- Maritim A. C., Sanders R. A and J. B. Watkins-III. Diabetes, oxidative stress and antioxidants a review. J Biochem Mole Toxicol. 2013;17:24-38.
CrossRef - Mee-Jung K., Song-Suk K and Soon-Dong K. Anti-diabetic effect of Red Ginseng-Chungkukjang with Green Laver or Sea Tangle. J Food Sci and Nutri. 2010;15:17.
- Nishimiki M., Rao N. A and Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem and Biophy Res Communi. 2014;46:849-853.
CrossRef - Oyaizu M. Studies on products of browning reactions: Antioxidant activities of products of browning reaction prepared from glucose amine. Japanese J Nutri. 2015;44:307-315.
CrossRef - Pari L and Latha M. Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singapore Med J. 2010;43:617-621.
- Prashanth K. V., Sashidhara S., Kumar M. M and Shidhara B. Y. Effect of Luffa echinata on lipid peroxidation and free radical scavenging activity. J Pharmacy and Pharmacol. 2010;52:891-894.
- Sushruta K., Satyanarayana S., Srinivas N and Sekhar J. R. Evaluation of the blood-glucose reducing effects of aqueous extracts of the selected Umbelliferous fruits used in culinary practices. Trop J Pharma Res. 2016;5:613-617.
- Thalapaneni N. R., Chidambaram K. A.,Ellappan T., Sabapati M. L and Mandal S. C. Inhibition of carbohydrate digestive enzyme by Talinum portulacifolium (Forssk) leaf extract. J Compl and Integ Med. 2010;5:1-10.
- Thambidurai M., Rajesh P., Balamurugan B and Kannan V. R. In vitro antioxidant and anti-microbial study on Cassia auriculata Linn. Int J Pharmacy and Biol Scie. 2010;2:1-7.
- Yamaguchi F., Ariga T., Yoshimura Y and Nakazawa H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agri and Food Chem. 2010;48:180-185.
CrossRef








