Chandrakant Waghmare1 and Sachin Kore2
1Department of Pharmacology, Ashwini Rural Medical College and Hospital, Solapur (India).
2Assistant Professor in Skin and V.D., K.B.N. Institute of Medical Sciences, Gulbarga (India).
Abstract
Tuberculosis is a major health problem afflicting the world, especially the third world countries. Multi-Drug Resistant Tuberculosis (MDR-TB) is defined as the disease caused by Mycobacterium Tuberculosis resistant to at least Isoniazid & Rifampicin , with or without resistance to other anti-TB drugs. Contrary to earlier belief, multi-drug resistant Mycobacteria have proved to be as infectious as drug susceptible ones & can cause severe disease. Erratic prescribing by physicians & patient non-adherence to the treatment regimens are the major causes leading to the emergence of MDR-TB. World Health Organization (WHO) has issued guidelines for the management of MDR-TB. The regimens for the treatment of MDR-TB are designed according to the availability / unavailability of drug susceptibility test results. Second line anti-Tuberculosis (anti-TB) drugs and the newer agents have vital role in treatment of MDR-TB. Directly Observed Therapy (DOT) is strongly recommended for all anti-TB regimens. In general, the treatment of MD R-TB is costlier, less effective & more toxic as compared to treatment of drug susceptible Tuberculosis. In last few years there has been increased incidence of what has been described as ‘Extensively Drug Resistant Tuberculosis (XDR-TB). A WHO consultation in held in March 2012 has come up with the diagnostic definition and various treatment options for the management of Extensively Drug Resistant Tuberculosis (XDR-TB).
Keywords
Drug Resistance; Tuberculosis; MDR-TB; XDR-TB
Download this article as:| Copy the following to cite this article: Waghmare C, Kore S. Multi-Drug Resistant Tuberculosis (MDR-TB) and its drug Treatment. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Waghmare C, Kore S. Multi-Drug Resistant Tuberculosis (MDR-TB) and its drug Treatment. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2422 |
Introduction
Tuberculosis (TB) continues to haunt mankind & remains a world wide health problem even today, despite isolation of its causative agent, advent of BCG Vaccine & availability of effective chemotherapeutic agents. According to WHO, one third of the world population is infected with Mycobacterium Tuberculosis. The disease is taking heavy toll on the third world countries of Asia & Africa. HIV epidemic & emergence of resistant forms of TB especially MDR-TB together have added a new dimension to the problem. It is well known that people living with HIV/AIDS are very susceptible to contracting TB as compared to general population. The incidence of MDR-TB is also higher in these patients. In the last two decades there have been a number of outbreaks of M D R-TB in United States & other parts of the world. These outbreaks were marked by very high mortality rates. WHO has taken cognizance of the problem. In fact the WHO declaration of TB as a “ Global Emergency” in 1993 was prompted by the emergence of M. D. R. –TB. The management of TB is getting even more complicated with the emergence of Extremely Drug Resistant TB (XDR-TB), a form of Multi Drug Resistant TB where the M. Tuberculosis is resistant not only to the first line anti TB agents but also to the second line anti TB agents.
Overview of the drugs used in the treatment of Mdr-tb
The anti-TB drugs are broadly classified into first line drugs and second line drugs based on their efficacy and toxicity.
First line drugs
Isoniazid ( INH ) , Rifampicin , Pyrazinamide , Ethambutol& Streptomycin are labeled as the first line drugs which have high efficacy & less adverse effects. These agents are routinely used for the treatment of TB. Isoniazid & Rifampicin are the two most potent drugs in the treatment of TB.
Second line drugs
The second line drugs have lower efficacy and are associated with more adverse effects in comparison to the first line agents & are therefore used in special circumstances such as for treatment of MDR-TB. This group includes Aminoglycosides such as Kanamycin, Amikacin and Capreomycin, Flouroquinolones such as Ofloxacin, Moxifloxacin, Levofloxacin, oral bacterostatic agents such as Ethionamide , Prothionamide, Cycloserine, Terizidone, p-aminosalicylic acid (PAS) and ‘Group Five’ drugs which includes Clofazimine, Linezolid, Amoxicillin/Clavulanate, Thioacetazone, Clarithromycin, and Imipenem.
Macrolide antibiotics
Drugs such as Azithromycin , Clarithromycin are used for the treatment of infections caused by atypical Mycobacteria.
Multi-drug resistant tuberculosis (Mdr-tb)
It is defined as the disease caused by M.Tuberculosis that is resistant to at least Isoniazid (INH) & Rifampicin, with or without resistance to other anti-TB drugs6. The drug treatment of MDR-TB is difficult as the options are limited owing to the resistance to INH & Rifampicin, two of the most potent anti-TB drugs.
Magnitude of the Problem
As the HIV infection & TB go hand in hand, emergence of MDR-TB has complicated the HIV epidemic. After declaring TB a Global emergency in 1993, WHO along with International Union Against TB & Lung Disease ( IUATLD ) had launched “Global Drug Resistance Surveillance Project” between 1994 & 19977.
The project revealed that the prevalence of primary multi-drug resistance to anti-TB drugs was 1.4 percent (Resistance to INH & Rifampicin with or without resistance to other drugs in patients who had no history of anti-TB treatment ) and that of secondary or acquired multi-drug resistance was 13 percent .(Resistance to INH & Rifampicin with or without resistance to other drugs in patients having history of anti-TB treatment ). Earlier, drug / multi-drug resistant strains of M. Tuberculosis were not considered as infectious as the drug susceptible strains, because the former showed less virulence in guinea pigs & poor growth in artificial media. It was thought that drug / multi-drug resistant TB is a problem only for the concerned individual & not for the community. But recent evidence suggests that drug / multi-drug resistant strains of M. tuberculosis are as infectious as their drug susceptible counterparts8 . It is also established that the drug / multi-drug resistant strains of M. Tuberculosis can cause very severe disease. Further, the treatment of MDR-TB is not as effective as the treatment of drug susceptible TB. In last few years there have been reports of what has been described as ‘Extensively Drug Resistant TB (XDR-TB)’. XDR-TB is a form of MDR-TB where the patients have resistance to Second Line anti- TB drugs as well. XDR-TB has drastically reduced the treatment options for the patients concerned.
Diagnosis / Detection of MDR-TB
Drug / multi-drug resistant TB should be suspected in patients if
Patient has taken treatment for Tuberculosis in the past.
INH resistance prevalence in community is more than 4 percent.
There is likelihood of patient being exposed to MDR-TB.
There is poor response to the drug treatment as indicated by prolonged fever or cough, sputum conversion failure despite four months of standard short course chemotherapy9 .
Mycobacteria grow slowly and hence drug susceptibility tests take about 10- 12 weeks. Thus rapid testing methods are necessary to detect drug resistance early & institute appropriate drug treatment. Slide culture sensitivity methods (detect drug resistance in about a week10, phage based method using the ‘Luciferase incorporated
Phage11-13, BACTEC method (Radiometric technique based on detection of radio labeled CO2 as a measure of growth index for Microorganisms14-15, genotypic techniques involving rapid genotypic analysis of Mycobacteria to detect gene mutations causing drug resistance16 are some of the rapid testing methods to detect drug resistance. Currently such rapid testing methods are beyond reach of most laboratories due to cost factor.
Treatment of Multi-drug Resistant TB (MDR-TB)
In general, the treatment of Multi-Drug Resistant TB is less effective. The cost of the treatment as well as the incidence of adverse effects is higher in comparison to the treatment of drug susceptible TB. The WHO has issued important guidelines for the management of drug / Multi-Drug Resistant TB.
WHO guidelines for the drug treatment of MDR-TB
While designing the regimen for MDR-TB patients
One shouldnot aim to keep drugs in reserve. Keeping the drugs in reserve won’t serve any purpose as treatment of MDR-TB with best possible drugs is the only chance of survival for the patient.
As for the choice of the drugs, those drugs, which the patient has not received before, should be used. This is because the bacilli are almost certain to be sensitive to such drugs.
The initial regimen should include alteast 3 drugs, preferably 4 or 5, effective against multi-drug resistant bacilli. ( Drugs which the patient has not received before).
It is desirable to include combination of an injectable aminoglycoside & Pyrazinamide among the drugs chosen to design the regimen because the combination is synergistic as far as bactericidal activity is concerned.Pyrazinamide may be chosen even if it has been used in the patient before because resistance toPyrazinamide is usually unlikely.
After sputum conversion one or more drugs may be withdrawn, especially the drug / drugs which is/ are toxic to the patient.
MDR-TB treatment should be continued for at least 18 months after the patient becomes sputum negative.
Treatment should be on daily basis & must be based on Directly Observed Therapy (DOT).
Drugs effective in MDR-TB(Table-II)
Drugs used in the management of MDR-TB can be classified according to their activity against M. Tuberculosis.
Bactericidal drugs: Aminoglycosides and Thioamides have very high bactericidal activity while Fluoroquinolones have low bactericidal action.
Bacteriostatic drugs (at usual therapeutic doses): E.g. Ethambutol, Cycloserineare also effective in the treatment of MDR-TB and therefore form part of anti-TB regimens.
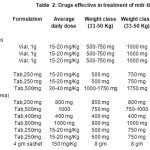 |
Table 2: drugs effective in treatment of mdr-tb |
WHO has recommended anti TB regimens for treatment of MDR-TB according to the availability / unavailability of drug susceptibility tests results
Regimen for MDR-TB patients when drugs susceptibility tests are unavailable: (Table III)]
 |
Table 3: Drug treatment of MDR-TB, before / without susceptibility test results. |
Pyrazinamide and at least 3 drugs, which the patient has never received before, are chosen for initial phase.
If drug susceptibility tests are not available even after sputum conversion , two best tolerated & more active drugs should be continued for 18 months.
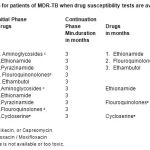 |
Table 4: Regimen for patients of MDR-TB when drug susceptibility tests are available: (Table IV) |
There is lack of reliable information on susceptibility ofM.Tuberculosis to Pyrazinamide. In case there is proven resistance to Pyrazinamide & if it correlates with clinical data Pyrazinamide may be replaced with Cycloserine or PAS. Usually resistance to Pyrazinamide is unlikely even if it has been used before in the patient. Regarding Rifampicin, there is lack of information on the effective regimens for patients in whom mono-resistance to Rifampicin is demonstrated. If the bacilli are resistant to Isoniazid & Rifampicin, a 5-drug regimen should be used for initial phase.
Patients of MDR-TB require second line drugs, which are less effective & have more adverse effects as compared to first line drugs. So the patient must be encouraged to tolerate the adverse effects. It should be imbibed upon the patient that compliance with the prescribed regimen alone can prevent the death. Psychological support to the patient is also of paramount importance.
Extremely Drug Resistant TB (XDR-TB)
WHO Consultation on the diagnostic definition and treatment options (21-22 March 2012)
Main conclusions
Although there is increased incidence of Tuberculosis (TB) patients with severe patterns of drug resistance which is posing a formidable challenge to the clinicians, a new definition of resistance beyond Extensively Drug-Resistant TB (XDR-TB ) is not recommended, owing to the technical difficulties with drug susceptibility testing (DST) of many anti-TB drugs, insufficient evidence to link such DST results to treatment outcomes of patients, and the lack of standardized DST methods for several anti-TB drugs.
Rapid drug susceptibility testing (DST) of Isoniazid and Rifampicin or of Rifampicin alone is recommended over conventional testing or no testing at the time of diagnosis of TB, subject to available resources.
The use of sputum smear microscopy and culture rather than sputum smear microscopy alone is recommended for the monitoring of patients with MDRTB during treatment.
In the treatment of patients with MDR-TB, a fluoroquinolone should be used.
In the treatment of patients with MDR-TB, a later-generation fluoroquinolone rather than an earlier-generation fluoroquinolone should be used. In the treatment of patients with MDR-TB, Ethionamide or Prothionamide should be used.
In the treatment of patients with MDR-TB, four second-line anti TB drugs likely to be effective (including a parenteral agent), as well as pyrazinamide, should be included in the intensive phase.
In the treatment of patients with MDR-TB, regimens should include at least Pyrazinamide, a fluoroquinolone, a parenteral agent, Ethionamide (or Prothionamide), and either Cycloserine or PAS (p-aminosalicylic acid) if Cycloserine cannot be used.
In the treatment of patients with MDR-TB, an intensive phase of at least 8 months’ duration is recommended.
In the treatment of patients with MDR-TB, a total treatment duration of at least 20 months is recommended in patients without any previous MDR-TB treatment. Antiretroviral therapy is recommended for all patients with HIV and drug-resistant TB requiring second-line anti-tuberculosis drugs, irrespective of CD4 cell-count, as early as possible (within the first 8 weeks) following initiation of anti-tuberculosis treatment.
Prospectus
Newer Rifamycins such as Rifabutin, Rifapentin, Rifalazil& newer quinolones like Levofloxacin,Moxifloxacin appear promising to deal with MDR-TB. There are reports suggesting the usefulness of b-Lactam antibiotics such as Imipenem, Amoxicillin – Clavulanic acid in treatment of MDR-TB. Linezolid is an oxazolidinone derivative, which acts by inhibiting early steps of protein synthesis in the Mycobacteria& is found to be useful in MDR-TB. Several other drugs such as interferon-g, recombinant human interleukin 2 have been reportedly useful in MDR-TB. Currently these drugs are under clinical trials. As the treatment of MDR-TB has been found to be less effective, resectional surgery has been advocated in selected patients of MDR-TB who have good respiratory reserve & in whom drug treatment alone is not working.
References
- David H L, Basis for lack of drug susceptibility of atypical Mycobacteria, Rev Infect Dis., 3: 878-84 (1981).
- Cole S.T., Brosch R. Parkhill J. et. Al, Deciphering the biology of M. Tuberculosis from the complete genome sequence. Nature.,393: 537-44 (1998).
- Telenti A, Genetics of Drug Resistant Tuberculosis, Thorax., 53: 793-7 (1998).
- Ramaswamy S., Musser J.M., Molecular genetic basis of antimicrobial agent resistance in M. tuberculosis: 1998 update, Tuber Lung Dis., 79: 3-29 (1998).
- Zhang Y., Telenti A., Genetics of drug resistance in M. Tuberculosis, In: Hatfull G, Jr . Jacobs W.R., edited ‘Molecular genetics of Mycobacteria’, Washington American Society for Microbiology, In press, Washington, (2000).
- Veen, J. Drug Resistant TB: Back to sanatoria, surgery and cod liver oil? EurRespir J., 8: 1073 (1995).
- Pablos Mendez A, Biokin N, Reider HL et al, Global Survellance for anti TB drug resistance, 1994-1997, N Engl. J. Med ., 338: 1641 (1998).
- Snider DE Jr, Kelley G.D,Cauthen G.H. et al, Infection & Disease among contacts of TB cases with drug resistant & drug susceptible bacilli, Am Rev Repir Dis., 132: 125 (1985).
- AmalioTelenti and Michael Iseman, Drug – Resistant Tuberculosis, Drugs, 59(2): (2000).
- Dickinson J.M., Allen B.W. Mitchison D.A. et al, Slide culture sensitivity tests, Tubercle .70: 115 (1989).
- Jacobs W. R., Barletta R. G., Udani R. et al, Rapid assessment of drug susceptibilities of M.Tuberculosis by means of luciferase reporter phages, Science 260: 819 (1993).
- Cooksey, R.C, Crawford J.T, Jacobs W.R. Jr et al, A rapid method for screening antimicrobial agents for activity against a strain of M. Tuberculosis expressing firefly luciferase Antimicrob Agent, Chemother.37: 1348 (1993).
- Mohammad Sadeghi and MojganYorahmadi.Orient. J. Chem. 27(3): 865-874 (2011).
- Good, R.C. and Mastro T. D, The modern mycobacteriology Laboratory: How it can help the clinician? Clin Chest Med ., 10: 315 (1989).
- Huebner, R.E., Good R.C, TokarsJ.Iet al, Results of a survey of state public health laboratories, J. ClinMycobacteriol., 31: 771 (1993).
- Telenti A, Imboden P, Marchesi F et al, Detection of Rifampicin resistance; mutations in M. tuberculosis. Lancet, 341: 647-50 (1993).
- WHO, http://www.who.int/tb/challenges/xdr/xdrconsultation/en/index.html accessed on Feb 7, (2012).







