Manuscript accepted on :01-Nov-2018
Published online on: 06-11-2018
Plagiarism Check: Yes
Reviewed by: Jagdish Joshi
Second Review by: Abdallah Hussien Fathy
Chinna Babu Pydi and Satyanarayana Rentala
and Satyanarayana Rentala
Department of Biotechnology, GITAM Institute of Technology, GITAM deemed to be University, Gandhi nagar campus, 530045, Visakhapatnam, India.
Corresponding Author E-mail: med.bio1979@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1560
Abstract
Angiogenesis research investigates the formation of new blood vessels in wound healing, tumour growth and embryonic development. The present paper describes the application of circulating endothelial progenitor (CEP) cells expressing CD133 and VEGFR2. To do so, the CEPs were isolated from human peripheral blood and expanded in an artificial niche using in a transwell plate. The migration, adhesion, homing and differentiation of CEPs towards prostate cancer stem cells were studied. This model is more suitable to study tumor metastasis for the development of novel therapeutics.
Keywords
Development; Endothelial Progenitor Therapeutics;
Download this article as:| Copy the following to cite this article: Pydi C. B, Rentala S. An Artificial Niche for Circulating Endothelial Cells During Tumor Angiogenesis Mediated by Prostate Cancer Stem Cells. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Pydi C. B, Rentala S. An Artificial Niche for Circulating Endothelial Cells During Tumor Angiogenesis Mediated by Prostate Cancer Stem Cells. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=23946 |
Introduction
The tumor initiation and progression of many cancers depends on the occurrence a small subpopulation of cells called cancer stem cells.1 The concept of cancer stem cells within highly differentiated tumor, is now gaining more importance during cancer diagnosis and treatment.2,3 These cells are considered to be having tumor-initiating capacity.4 We have recently shown that a rare cell population in human prostate cancer, defined by the phenotype CD133+/α2β1 hi (high expression of α2β1 integrin) and comprising less than 0.1% of the tumor mass, has many of the properties of cancer stem cells.5,7 These properties include self renewal, extended lifespan, drug resistance, a high invasive capacity, a primitive epithelial phenotype and an ability to differentiate to tumors.6,7 Circulating Endothelial progenitor (CEP) cells are primarily occur in peripheral blood. CEPs were recently isolated from adult species; once adhered, these cells would differentiate in in vitro. To determine the role of CEPs contributing to tumor angiogenesis, we established a novel three dimensional model in association with prostate cancer stem cells to study tumor metastasis.
Methodology
Isolation of Circulating Endothelial Progenitor Cells (Ceps) from Peripheral Blood
All the studies were approved by Institutional Ethics Committee (IEC) of GITAM deemed to be University. Prostate cancer patients whose prostate specific antigen levels are more than 4 were selected and included in the study. Mononuclear cells were isolated using density-gradient centrifugation with Ficoll-Paque from 20 mL of peripheral blood of the patients. Immediately after isolation, 4×106 mononuclear cells were plated on 24-well culture dishes and maintained in endothelial basal medium and 20% FCS. After 24 hours in culture, non-adherent cells were removed by a thorough washing with PBS.
From the above cell population, CD133-positive and VEGFR2-positive cells were obtained by positive selection with CD133 and VEGFR2 immunomagnetic microbeads using an auto–magnetic cell sorting cell separation device. To detect the expression of circulating endothelial progenitor marker proteins, CEPs were incubated for 15 minutes with florescent-labeled monoclonal antibodies against human CD133 (conjugated with fluorescein isothiocyanate) and VEGFR2 (conjugated with phycoerythrin). The cells were fixed in 4% paraformaldehyde. Two-color flow cytometric analysis (BD-Accuri C6, USA) was performed using a flow cytometer.
Isolation of Prostate Cancer Stem Cells
Cancer cells were isolated from the prostate tissue of cancer patients (prostate-specific antigen levels > 4 ng mL−1) who were between 60 and 80 years of age. Biopsy samples from the prostate cancer patients (n = 10) were digested with a trypsin collagenase mixture to dissociate the cells. The CD133+ cells were selected using immuno-magnetic beads and analysed for the presence of CD133. The purity of CD133+ in the sorted samples was always higher than 94%. The CD133+ cells were also analyzed for CD44 expression. The immune-labelling of CD133 was performed using a monoclonal CD133 antibody conjugated to fluorescein isothiocyanate (FITC). The expression profile of CD44 in these cells was studied by flow cytometry using a phycoerythrin-labelled rabbit antihuman CD44 antibody.
Development of Three Dimensional Culture System
Iscove’s modified Dulbecco’s medium (IMDM), supplemented with antibiotic–antimycotic solution, was used to culture circulating endothelial progenitor cells. Serum-free medium (SFM) was prepared by supplementing IMDM with 2 mg/ml bovine serum albumin (BSA), antibiotics and cytokines (IL-3, IL-6, Insulin-Transferrin-Selenium) of final concentrations 50 ng/ml stem cell factor and 20 ng/ml Vascular Endothelial Growth Factor (VEGF). A Transwell plate was coated with a mixture of fibronectin (100 µg/ml) and laminin (50 µg/ml) for 4 hours. Excess solution was removed and the plate was air-dried for 2 h at 370C. CEPs derived as above were cultured at a density of 0.5×104 cells in each well in 500 µl SFM in a CO2 incubator.
Transwell plates were inserted in the 12-well plate containing prostate cancer stem cells (CD133+CD44+). The migration of CEPs from three dimensional matrix (fibronectin-laminin matrix) towards prostate cancer stem cells were studied using migration studies.
Studies on Migration and Differentiation of Endothelial Progenitor Cells Towards Prostate Cancer Stem Cells
Endothelial Cell Tube Formation Assay
All procedures were performed in a biological safety cabinet using aseptic technique to prevent contamination. We seeded red Dil (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine Perchlorate) labeled CEPs in Transwell plates in both 2D (Transwel plate without coating with fibronectin-laminin) and 3D culture systems in medium at 0.5×104 cells in the presence of prostate cancer stem cells (CD133+ CD44+ cells) in the lower chamber. For every 24 hours the number of endothelial progenitor cells migrated towards prostate cancer stem cells were enumerated using florescent microscopy. After 4 to 18 hours, the endothelial tube formation was also examined using fluorescent microscope by staining endothelial markers such as CD31 and vW factor (Von Willebrand factor).
The migrated CEPs were differentiated into endothelial cells to make a tube like structure. The differentiation of CEPs towards CD31 and Von Willebrand factor (vWF) was analysed using flow cytometer. FITC-labelled CD31 and PE-labelled vWF were used to stain the differentiated cells.
Results
Isolation of Circulating Endothelial Progenitor Cells (Ceps) from Peripheral Blood
CEPs were isolated from peripheral blood derived mononuclear cells using magnetic separation columns. Magnetic beads for CD133 and VEGFR2 were used. In all the experiments, the % purity of CEPs was 93%. As shown in Figure 1 CD133 positive cell population was first isolated from peripheral blood (as shown Figure 1B). VEGFR2-positive cell population was isolated from the above CD133-positive cells using magnetic columns. The percentage purity was always maintained around 93% (as shown Figure 1C). The Isotype IgG-FITC and Isotype IgG-PE were routinely used as control florescent labeling (as shown Figure 1A).
 |
Figure 1: Isolation of circulating endothelial cells from peripheral blood:
|
Figure 1A represent Isotype IgG controls for FITC and PE. Figure 1B indicates the percentage purity of CD133 cells isolated using immune-magnetic beads. Figure 1C indicates the percentage purity of CEPs (CD133 and VEGFR2 – positive cells) in peripheral blood mononuclear cells.
Isolation of Prostate Cancer Stem Cells
Prostate cancer stem cells were isolated from prostate cancer tissues using magnetic separation columns. Magnetic beads for CD133 and CD44 were used. In all the experiments, the % purity of CEPs was 95%. As shown in Figure 2 CD133 positive cell population was first isolated from prostate cancer tissue. CD44-positive cell population was isolated from the above CD133-positive cells using magnetic columns. The percentage purity was always maintained around 93% (as shown Figure 2B). The Isotype IgG-FITC and Isotype IgG-PE were routinely used as control florescent labeling (as shown Figure 2A).
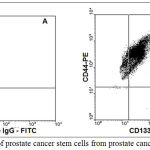 |
Figure 2: Isolation of prostate cancer stem cells from prostate cancer tissues:
|
Figure 2A represent Isotype IgG controls for FITC and PE. Figure 2B represents the percentage purity of prostate cancer stem cells (CD133 and CD44 positive cells) isolated from prostate cancer tissues using immune-magnetic beads.
Migration and Differentiation of Endothelial Progenitor Cells Towards Prostate Cancer Stem Cells
Endothelial cells seeded in 2D and 3D Transwell plates were cultured in presence of prostate cancer stem cells in lower chamber. The migration assay was performed by enumerating the endothelial progenitor cells migrated towards prostate cancer stem cells using Dil dye staining. The number of red color stained cells (CEPs) migrated towards prostate cancer stem cells were counted using florescent microscope. The % of CEPs migration in 3D Transwell plate was always better than 2D Transwell plate (Figure 3A). Once CEPs migrated towards prostate cancer stem cells, CEPs were differentiated into endothelial cells. This is a crucial step to make tube like structure to surround prostate cancer stem cells for cancer development. The percentage of differentiation in 2D and 3D culture system were investigated. The proposed 3D culture system (Figure 3C) showed better differentiation of CEPs into endothelial cells than 2D culture system (Figure 3B). This was calculated as the percentage of CEPs (CD133+VEGFR2+) differentiated into endothelial (CD31+vWF+) cells (Figures 3B and 3C). The tube like structure formation was also studied microscopically by staining CD31 (labeled with FITC) and vWF (labeled with PE) as shown in Figure 4.
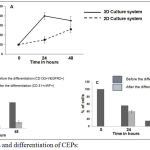 |
Figure 3: Migration and differentiation of CEPs:
|
Figure 3A represents the percentage migration of CEPs towards prostate cancer stem cells in Transwell migration chambers (2D and 3D). Figure 3B represents the differentiation of CEPs into endothelial cells in 2D culture system. Figure 3C represents the differentiation of CEPs into endothelial cells in 3D culture system.
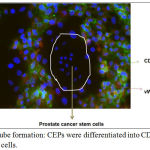 |
Figure 4: Endothelial tube formation: CEPs were differentiated into CD31 and vWF expressing endothelial cells.
|
The differentiated endothelial cells surrounded prostate cancer stem cells for further cancer development.
Conclusion
Circulating endothelial progenitor cells and prostate cancer stem cells were isolated from peripheral blood and prostate cancer tissues. 3D cultured CEPs migrated towards to prostate cancer stem cells better than 2D cultured CEPs. The differentiation of CEPs into endothelial cells was always better in 3D than in 2D system. The designed 3D tumor angiogenesis model will surely serve to develop novel therapeutics for the treatment of cancer.
Conflict of Interest
There is no conflict of interest.
References
- Rentala S., Balla M. M. S., Khurana S., Mukhopadhaya A. MDR1 gene expression enhances long-term engraftibility of cultured bone marrow cells. Biochemical and Biophysical Research Communications. 2005;335:957-964.
CrossRef - Gopal N. V., Rentala S., Roy S., Wadhwa R., Sharma S., Roychaudhury P. K., Mukhopadhyay A and Ray A. R. An Artificial Niche for Expansion of Long-Term Engraftable Hematopoietic Cells. Journal of Stem Cells. 2008;3(4):245-254.
- Rentala S & Narasu L. M. Oct 4 Expression Maintained Stem Cell Properties in Prostate Cancer Derived CD 133 MDR1 Cells. Tropical Journal of Pharmaceutical Research. 2009;8(1):3-9.
CrossRef - Rentala S., Devi P. Y & Narasu L M. α1 and β1 integrins enhance homing and differentiation of cultured prostate cancer stem cells. Nature Publishing Group – Journal of Andrology. 2010;12(4):548-55.
CrossRef - Collins A.T and Maitland N. J. Prostate cancer stem cells. Eur. J. Cancer. 2006;42:1213-1218.
CrossRef - Anne T. C., Paul A. B.,Hyde C ., Michael J. S and Norman J. M. Prospective Identification of Tumorigenic Prostate Cancer Stem Cells. Cancer Research. 2005;65:10946-10951.
CrossRef - Rentala S., Chintala R., Guda M.,Chintala M.,Lakshmi A.K., Narasu L. M. Atorvastatin inhibited Rho-associated kinase 1 (ROCK1) and Focal Adhesion Kinase (FAK) mediated adhesion and differentiation of CD 133 CD 44 prostate cancer stem cells. Biochemical and Biophysical Research Communications. 2013;441(3):586–592.
CrossRef







