Manuscript accepted on :
Published online on: 09-12-2015
Plagiarism Check: Yes
Ndubuisi N. Nwobodo¹*, Paul O. Okonkwo² and Nicholas C. Obitte³
¹Department of Pharmacology and Therapeutics, Ebonyi State University (Abakaliki). ²Department of Pharmacology and Therapeutics, University of Nigeria Teaching Hospital, Ituku (Ozalla). ³Department of Pharmaceutical Chemistry, University of Nigeria (Nsukka).
Abstract
This study is aimed at developing and describing a simple, precise, reliable and novel spectrophotometric technique for the determination of serial serum concentration of simvastatin in vivo. Double beam ultraviolet spectrophotometer is employed to determine absorbance readings for corresponding serial concentration of standard stock solution. The absorbance of test sample obtained from serum is then determined and the concentration of test sample deduced from the calibration curve. The calibration curve of simvastatin is found to be linear within Beer-Lambert’s law limits at concentration range of 0.1-20µg/ml. This is represented mathematically in the linear regression equation, y = 0.0174x-0.016. The maximal absorbance wavelength (lmax) is shown to be 280nm. The spectrophotometric technique described in this study has been shown to be quite appropriate, accurate and highly sensitive in detecting very low serum concentrations of simvastatin in vivo; and can be routinely employed clinically for therapeutic drug monitoring.
Keywords
Absorbance; concentration; simvastatin; therapeutic drug monitoring
Download this article as:| Copy the following to cite this article: Nwobodo N. N, Okonkwo P. O, Obitte N. C. Novel Extractive Ultraviolet Spectrophotometric Determination of Serum Simvastatin Concentration. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Nwobodo N. N, Okonkwo P. O, Obitte N. C. Novel Extractive Ultraviolet Spectrophotometric Determination of Serum Simvastatin Concentration. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2392 |
Introduction
Simvastatin, a 3-hydroxy-3-methyl glutaryl co-enzyme A reductase inhibitor, is chemically designated as [(1S, 3R, 7R, 8S, 8aR)] -8-[2-[2R, 4R) -4-hydroxy-6-oxo-oxan-2-2yl [-3, 7-dimethyl -1, 2, 3, 7, 8, 8a–hexahydronaphthalen-1-yl]2, 2-dimethyl butanoate1. It belongs to a class of drug known as statins that exert their effect by reduction of LDL levels through a mevalonic acid-like moiety that competitively inhibits HMG-CoA reductase.
Simvastatin is well tolerated and quite effective in the treatment of dyslipidemia and prevention of ischemic heart disease. The role of simvastatin in reducing mortality and incidence of coronary events has been well documented2. Hypercholesterolemic individuals treated with simvastatin showed a reduction in the levels of pro-inflammatory cytokines3. Simvastatin has been found to reduce inflammation, decrease leukocyte rolling and adherence in apolipoprotein-E deficient mice4,5. There is need for therapeutic drug monitoring in the course of simvastatin therapy. There are various methods reported in the literature for determination of simvastatin such as high performance liquid chromatography with ultraviolet detection6, liquid chromatography coupled with tandem mass spectroscopy7 and ultraviolet spectrophotometry8. Other analytical methods have also been reported for determination of simvastatin9-11. However, these techniques are mostly employed in the determination of simvastatin in bulk and pharmaceutical dosage forms. Those that can be employed in the determination of simvastatin in serum or biological fluids, are not suitable for routine analysis because they require sophisticated instruments that are not readily available, involve numerous steps and tedious processes resulting to insufficient sensitivity. The review of literature revealed that no method has been reported for determination of simvastatin in serum or biological fluids using ultraviolet spectrophotometry. This study attempted to develop and describe a simple, precise, reliable and novel extractive ultraviolet spectrophotometric technique for the determination of serial serum concentration of simvastatin.
Materials and Methods
Patient Selection
Ten healthy volunteers are selected after explaining the rationale of study and informed consent obtained. The age range of participants varies from 21 to 48 years and male/female ratio given as (2:3). Baseline serum liver transaminases and creatine kinase assays are carried out prior to administration of simvastatin. This was done to monitor the occurrence of adverse effects associated with use of simvastatin which manifests as elevation in the serum liver enzymes. Simvastatin is administered in the dose of 0.6mg/kg/day. Blood samples are collected for analysis before and after administration of drug.
Instrumentation
The UV 754 double beam spectrophotometer equipped with 809C52 microcomputer for memory storage function, RS-232 integrated interface and special auto-collinating optical system is used. The spectral bandwidth is £6nm and wavelength range 200-800nm. The transmittance range is 0.0-100.0%(T) while T-A conversion accuracy is ±0.002(A).
Reagents
Simvastatin (10mg) made by Ranbaxy Laboratories Limited, India and reagents of analytical grade are used. These include: 95% methanol, 99% ethanol, 2% chloroacetic acid, concentrated nitric acid, concentrated hydrochloric acid, concentrated sulphuric acid, tetrahydrofuran (THF), perfluoroctanoic acid (HPFOA) and acetonitrile.
Extraction of Pure Simvastatin from Tablets
20 tablets from the sample of 10mg simvastatin are weighed, crushed and powdered in a mortar. 200ml of 95% methanol v/v is introduced into the crushed tablets and stirred. The mixture is vigorously shaken and filtered into a beaker with the use of a filter paper. The filtrate is further re-filtered and the final filtrate emptied into a clean stainless steel plate and allowed to evaporate. After complete evaporation over a hot plate at 100oC, the drug is left behind as crystals which are collected and stored at ambient temperature under shade.
Preparation of Standard Stock Solution
10mg of simvastatin was taken from the pure sample and dissolved in 100ml of a mixture of 95% v/v of methanol to obtain the stock solution. The stock solution was suitably diluted to obtain concentrations in the range of 0.1 to 20µg/ml
A sample solution is initially scanned spectrophotometrically to determine the wavelength of maximum absorption for simvastatin. The scan result showed 280nm to be the appropriate wavelength of maximum absorption and this is used for subsequent readings.
Extraction of Simvastatin in Serum
2ml fresh anti-coagulated blood samples using EDTA as anti-coagulant are collected before oral administration of drug and then 2, 12 and 24 hours after. The fresh blood samples are centrifuged at 4000 rpm for 5 minutes to get the plasma. The plasma is deproteinated by treating with 2% v/v chloroacetic acid. The resultant precipitate is filtered and the filtrate collected. To each filtrate is added 2ml of perfluoroctanoic acid (HPFOA), 2ml of tetrahydrofuran (THF), 2ml concentrated nitric acid and subjected to centrifugation at 4000 rpm for 15 minutes. The sedimented liquid phase is collected by a micro-syringe. A stable colour formation occured in the presence of concentrated nitric acid. The absorbance of colour product, that is the test sample, is then determined and corresponding concentration deduced from the graph.
Recovery Studies
The developed method was validated by performing recovery studies at 80%, 100% and 120% of the test concentration in line with approved guidelines (USP 2006).
Results
The concentration of simvastatin and the corresponding absorbance readings are given in Table 1. Table 2, shows the optical characteristics of simvastatin revealing maximal absorbance wavelength (λmax) of 280nm. The value 0.0174 represents the slope of the graph, b; while the value -0.016 represents the interception of the linear graph on y-axis c, at the value of x=0.
Table 1: Calibration Of Ultraviolet Spectrophotometric Determination Of Simvastatin
| Absorbance (A) | Concentration (μg/ml) |
| 0.007 ± 0.001 | 0.1 ± 0.04 |
| 0.016 ± 0.001 | 0.5 ± 0.04 |
| 0.033 ± 0.001 | 1.0 ± 0.04 |
| 0.049 ± 0.001 | 5.0 ± 0.04 |
| 0.074 ± 0.001 | 10.0 ± 0.04 |
| 0.091 ± 0.001 | 20.0 ± 0.04 |
Table 2: Optical Characteristics Of Ultraviolet Spectrophotometric Determination Of Simvastatin
| Optical Characteristic | Measurements |
| Regression Equation, y = bx + c: | |
| Slope (b) | 0.0174 |
| Intercept (c) | -0.016 |
| Λmax | 280nm |
| Correlation Coefficient (R2) | 0.984 |
| Linearity Range | 0.1-20μg/ml |
| Absorbance Range | 0.0-3.0 (A) |
The calibration curve of simvastatin as shown in Figure 1, is constructed by plotting the absorbance of simvastatin (y) against the concentration (x). It is found to be linear within Beer-Lambert’s law limits at concentration range of 0.1-20μg/ml. A six point calibration curve for the reference standard is obtained. The calibration areas are drawn based upon the Beer-Lambert’s law that absorption is proportional to concentration. A plot of absorbance against concentration; keeping the path-length constant gives a straight line.
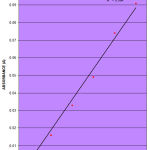 |
Figure 1
|
This is expressed mathematically in the linear regression equation given as:
y = 0.0174x – 0.016.
As stated in Beer-Lambert’s law, absorbance (y) is directly proportional to
concentration (x): Thus: y µ x (y= bx)
Where b= slope of the graph. Transforming the subject of equation: x= y/ b.
As shown in the linear regression equation above, b= 0.0174. Hence, when absorbance of drug solution, y is known, the concentration, x is readily computed.
Discussion
Different organic solvents including ethanol, methanol, acetonitrile and tetrahydrofuran (THF) are used to develop spectrophotometric determination of simvastatin in different solutions. Methanol showed the greatest colour development, satisfactory stability and reproducibility among the organic solvents used. Consequently, methanol is selected as the organic media for ultraviolet spectrophotometric determination of simvastatin. Concentrated nitric acid is found to be most efficient in eliciting colour development than other acid solutions. The study reveals that addition of tetrahydrofuran resulted in the highest possible extraction of simvastatin compared to other organic solvents. Optimal centrifugation time and speed determined in the study reveal that a centrifuge speed of 4000 rpm for 15 minutes resulted in better quantitative extraction of simvastatin and is employed throughout the study.
A study reported mean serum levels of simvastatin given as 0.4µg/ml 4 hours after oral administration, and less than 10% of peak remained after 12 hours12. Simvastatin is a lactone pro-drug that is hydrolyzed to the active corresponding β-hydroxy acid, which can be re-converted back to the lactone13. Pharmacokinetic study has reported that simvastatin and their active species are highly bound (95%) to plasma proteins being very hydrophobic14. Similar studies have reported mean half life of 1.9 hours and mean peak plasma time above 1.4 hours15,16. The low concentrations and short half-lives in plasma could be explained in part by the rapid uptake of the drugs by the liver, the major site of cholesterol biosynthesis in humans. A study for determination of simvastatin in pharmaceutical dosage form using RP-HPLC method showed linearity at concentration range of 2 to 10µg/ml17. A similar study employed RP-HPLC method and reported linearity range of 0.5 to 20µg/ml within Beer-Lambert’s law limits9. Another study employing ultraviolet spectrophotometric method for determination of simvastatin obeyed Beer-Lambert’s law within the concentration range of 4 to 16µg/ml18. Interestingly, the simvastatin calibration curve in the developed method as shown in present study was found to be linear within concentration range of 0.1 to 20µg/ml. Hence, the developed method is as accurate and precise as RP-HPLC in detecting very low concentrations of simvastatin in serum. However, it is less expensive, time-consuming and cumbersome. The linearity range of 4-16µg/ml reported for another ultraviolet spectrophotometric method is quite narrow and unsuitable for application in determining simvastatin in serum or biological fluids. It is shown, however, that when a known amount of the drug solution is added to the powdered sample and subjected to an estimation by the same method, there is a high recovery indicating that the developed method for determination of simvastatin is highly accurate and reliable.
In conclusion, the ultraviolet spectrophotometric technique described in this study is quite accurate, precise, highly sensitive and validated in the determination of simvastatin in serum or biological fluids. It can be routinely employed clinically for therapeutic drug monitoring (TDM) in the course of simvastatin therapy.
Acknowledgments
I wish to acknowledge Dr. S.A. Igwe, Associate Professor/Head, Department of Pharmacology and Therapeutics, ESUT College of Medicine, Parklane for his useful advice. Also acknowledged is Mr E.O. Eze, Chief Laboratory Technologist, Department of Pharmacology and Therapeutics, UNEC for his assistance.
Reference
- Martindale-The Extra Pharmacopoeia. The Royal Pharmaceutical Society, London. 30th, pp. 990-991 (1993).
- The 4S Investigators. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease. The Scandinavian Simvastatin Survival Study (4S). Lancet. 344: 1383-1389 (1994).
- Ferro D. and Violi F. Simvastatin inhibits the monocyte expression of pro-inflammatory cytokines in patients with hypercholesterolemia. Am. Coll. Cardiol. 36: 427-431 (2000).
- Sparrow C.P., Burton A., Hernandez M., Mundt S., Hassing H., Patel S. and Rosa R. Simvastatin has anti-atherosclerotic activities independent of plasma cholesterol lowering. Arteroscler. Thromb. Vasc. Biol. 21: 115-121 (2001).
- Scalia R., Gooszen M.E., Jones S.P., Hoffmeyer M., Rimmer D.M., Trocha S.D., Huang P.L., Smith M.B., Lefer A.M. and Lefer D.J. Simvastatin exerts both anti-inflammatory and cardio-protective effects in apolipoprotein-E deficient mice. 103: 2598-2603 (2001).
- Alvarez-Luge A., Valenzuela C., Squella J.A. and Nunez-Vergara L.J. Stability study of simvastatin under hydrolytic conditions assessed by liquid chromatography. J. AOAC Intl. 88: 1631-1636 (2005).
- Wang L. and Asgharnejad M. Second derivative ultraviolet spectrometric determination of simvastatin in its tablet dosage form. Pharm. Biomed. Anal. 21: 1243-1248 (2000).
- Vuletic M., Cindric M. and Koruznjak J.D. Identification of unknown impurities in simvastatin substance and tablets by liquid chromatography/tandem mass spectrometry. Pharm. Biomed. Anal. 37: 715-721 (2005).
- Neelima B., Kumar P.R., Krishna M.M., Bindu V.H. and Prasad Y.R. Simultaneous estimation of simvastatin and ezetimibe by RP-HPLC in pure and pharmaceutical dosage form. Oriental Journal of Chemistry. 24(1): 195-200 (2008).
- Ashfaq M., Ullakhan , Qutab S.S. and Naeemrazzaq S. HPLC determination of ezetimibe and simvastatin in pharmaceutical formulations. J. Chil. Chem. Soc. 52(3): 1220-1223 (2007).
- Backett A.H. and Stenlake J.B. In: Practical Pharmaceutical Chemistry. Part 2, CBS publishers and distributors. 4th, p. 282 (2002).
- Vickers S., Duncan C.A., Chen I.W., Rosegay A. and Duggan D.E. Metabolic disposition studies on simvastatin, a cholesterol-lowering pro-drug. Drug Metab. Dispos. 18: 138-145 (1990).
- Corsini A., Bellosta S., Baetta R., Fumagalli R. and Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Ther. 84: 413-428 (1999).
- Pentikanen P.J., Saraheimo M., Schwartz J.I., Amin R.D., Shwartz M.S., Brunner-ferber F. and Rogers J.D. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in humans. Clin. Pharmacol. 32: 136-140 (1992).
- Desager J.P. and Hormans Y. Clinical pharmacokinetics of 3-hydroxy-3-methyl glutaryl co-enzyme A reductase inhibitors. Pharmacokinet. 31: 348-371 (1996).
- Mauro V.F. Clinical pharmacokinetics and practical applications of simvastatin. Pharmacokinet. 24: 195-202 (1993).
- Dixit R.P., Barhate C.R., Padhye S.G., Viswanathan C.L. and Ngarsenker M.S. Stability indicating RP-HPLC method for simultaneous determination of Simvastatin and ezetimibe from tablet dosage form. Indian Journal of Pharmaceutical Sciences. 72(2): 204-210 (2010).
- Balaji S. and Sunitha A. Development and validation of spectrophotometric method for simultaneous determination of simvastatin and ezetimibe in tablet formulations. J. Pharm. Sci. 23(4): 375-378 (2010).







