Mostafa Soleimannejad Najafabadi1 and Mohammad Taghi Khorasani2
1Department of Biomedical Engineering, Science and Research Branch, Islamic Azad University, Tehran (Iran).
2Department of Biomaterials, Iran Polymer and Petrochemical Institute, Tehran (Iran).
Abstract
Poly urethane as a biomaterial used for medical applications. In this study, polyurethane surface grafted with to acrylamide by oxygen plasma radiation. Grafted surface were investigated by ATR-FTIR, SEM, Zeta potential and cell culture analyses. ATR-FTIR results and SEM images showed presence of acrylamide groups and successful grafting on the polyurethane surface. Cellular culture results showed more adhesive and proliferate of fibroblast cells on the acrylamide grafted polyurethane surface than the control groups. The acrylamide grafted polyurethane surface can be used for tissue engineering and medical applications.
Keywords
Polyurethane; Acrylamide ; Grafting ; Plasma radiation; Surface modification
Download this article as:| Copy the following to cite this article: Najafabadi M. S, Khorasani M. T. Surface modification of polyurethane with acrylamide by plasma radiation and its cellular investigations. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Najafabadi M. S, Khorasani M. T. Surface modification of polyurethane with acrylamide by plasma radiation and its cellular investigations. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2317 |
Introduction
Polyurethane (PU) is a hydrophobic polymer without bioactive fragments, which might limit its in vivo application. To achieve positive cell–biomaterial interaction, functional groups or biomacromolecules have been immobilized onto polymer for direct tuning of surface without altering their bulk properties.1-6 Biomaterials wettability is an important factor in the surface modification of materials. Surface modification of hydrophobic polymer surfaces can be achieved by wet (acid, alkali), dry (plasma) and radiation treatments (ultraviolet radiation and laser).7-10 Non thermal and low pressure plasma have been used in a series of surface treatment applications. The majority of plasma-assisted technologies are based on low pressure processes.11 The treatment of polymeric materials with plasma is a frequently-used technique to accomplish surface modifications that affects chemical composition as well as surface topography. Moreover, microwave discharges are routinely employed in the processing of materials to deposit films as well as coatings.12,13 In this work , the modified polyurethane were obtained with acrylamide grafting through the plasma radiation method with oxygen gas. The samples were evaluated by attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), scanning electron microscope (SEM), and zeta potential, and also the cell culture with fibroblast cells
Materials and methods
Twenty five ml solution of dimethylformamide (Sigma Co.) poured in 50 ml tube then 2.5 g polyurethane (Bayer Co.) added to the solution and was well stirred for 12 h in order to obtained homogeneous solution. The solution poured in Petri dish and kept at 25°c for a few days to remove the solvent. In our current experimental observation, we used a plasma source (K1050x and Emitech) to modify the surface of polyurethane samples. The samples were placed in plasma chamber and exposed to oxygen gas for 60, 120 and 180s. The irradiated samples at various times were investigated by structural analysis and microscopic investigations. The plasma surface treatment was reached in induced plasma with surface wave at power level of 60 W. The acrylamide solution with to distinctive concentration were poured in a baker, then the modified polyurethane samples were placed in and immersed in the acryamide solution. The samples degassed by nitrogen. This process was followed to increase the efficiency of the free radical polymerization, Then, the samples were brought out and washed by distilled water and were put in distilled water for 72 hours and Soxhleted for removing the ungrafted monomer, then were taken out for analysis
ATR – FTIR Analysis
For surface identification of modified samples, the samples were studied before and after surface modification by an infrared spectrometer device (Bruker IFS 48). For this study, the samples must be cleaned before being used in the study.
Scanning electron microscopy
The surface characteristics of various grafted and ungrafted films were studied by scanning electron microscopy (SEM; Cambridge Stereo-scan, model S-360; Cambridge Instruments, Wetzlar, Germany) to analyze the changes in the surface morphology. The films were first coated with a gold layer (Joel fine coat, ion sputter for 2 hours) to provide surface conduction before their scanning.
Zeta potential study
Zeta potential of surfaces was studied by Anton Paar analyzer. The samples were sectioned in distinctive dimension (thickness: 300 micron; length:40 mm and width:20 mm) and analyzed at 25°c and Ph:6.5-7.
Cellular study
For cellular analysis, fibroblast cell suspension (L929) from mouse tails was prepared according to International Organization for Standardization 10993 standards. The polyurethane surfaces were well cleaned and sterilized. Individual samples were placed in Petri dishes using a sterilized pincer; 3 cc of the cell suspension was removed by pipette and poured into the control and experimental samples. Thereafter, all of the samples were placed separately in a Memmert incubator at 37°C for 48 hours. The samples in the polystyrene Petri dish were removed from the incubator after 48 hours and studied using an Eclipse TS-100 photonic microscope (Nikon, T-B 2.5x, Japan).
Results
ATR-FTIR Study
ATR-FTIR spectra results of the un-grafted and acrylamide grafted polyurethane samples have been shown in Figure 1. The ATR-FTIR spectrums of polystyrene surface have been shown below Figure 1a. The Polyurethane picks characteristics include 3300-3500 cm-1 indicating primary amine groups and 1690-1730 cm-1 indicating C=O groups and 2800-3000 cm-1 indicating CH3 groups. All these picks are found in acrylamide grafted polyurethane samples but secondary amine groups indicated 3300-3500 cm-1 for the acrylamide grafted samples (Figure 1b). This conclusion shows grafting between the acrylamide and the polyurethane surface occurs by activation of plasma radiation.
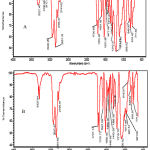 |
Figure 1: Spectra of ATR-FTIR of normal polyurethane (A), Acrylamide grafted polyurethane at 180 s (B). |
Surface morphology study
Figures 2 show SEM images from the un-grafted polyurethane and plasma modified samples and the grafted sample in the different radiation times. The figure 3 shows the surface morphology (cross-section) for the grafted sample in the different radiation times. The SEM image of un-treated polyurethane sample shows white spots and surface roughness due pollution and production process. The figure 2b shows the smooth surface morphology for the plasma modified polyurethane. The figures 2c-e show surface roughness due acrylamide grafting on the plasma modified polyurethane. The graft thickness average for the grafted samples obtained about 8 micrometer that grafting showed in figure 3.
 |
Figure 2: Scanning electron microscopy of untreated polyurethane (a) oxygen-plasma modified polyurethane(b) acrylamide grafted polyurethane surface at different radiation times 60s (c) ; 120 s (d) and 180 s (e). MAG:3000X . |
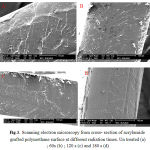 |
Figure 3: Scanning electron microscopy from cross- section of acrylamide grafted polyurethane surface at different radiation times. Un treated (a) ; 60s (b) ; 120 s (c) and 180 s (d). |
Zeta potential results
Table 1 shows zeta potential of the un-grafted polyurethane and plasma modified samples and the grafted sample. The zeta potential of the un-grafted polyurethane obtained -18.59 mv and for the plasma modified samples about -26 mv. This result demonstrated presence of oxide and peroxide negative groups on the oxygen plasma modified polyurethane surface. The grafted polyurethane samples have positive potential than the other samples due acrylamide grafting on the polyurethane surface.
Table 1: zeta potential of the un-grafted and plasma modified and the grafted samples.
|
grafted PU |
plasma modified PU |
un-grafted PU |
Sample |
|
-16.53 |
-25.89
|
-18.59
|
Zeta potential (mv) |
Cellular results
Figure 4 shows cellular assay for TCPS (control, 4a), un-treated PU film (4b), plasma modified at different times (60, 120 and 180 s) and acrylamide grafted PU surface. The results showed high viability for plasma modified and acrylamide grafted samples than un-treated polyurethane. The modified samples with plasma in longer times showed a better viability. Cellular Images showed high cell growth on the grafted surfaces, especially those grafted with active gas in longer times.
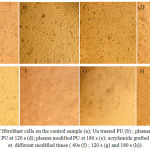 |
Figure 4: The culture of fibroblast cells on the control sample (a); Un treated PU (b) ; plasma modified PU at 60 s (c); plasma modified PU at 120 s (d); plasma modified PU at 180 s (e); acrylamide grafted polyurethane surface at different modified times ( 60s (f) ; 120 s (g) and 180 s (h)). |
Conclusion
In this study, polyurethane surface modified with to oxygen plasma radiation and acrylamide and investigated by different analyses. The ATR-FTIR and SEM and zeta potential analyses well demonstrated presence of acrylamide grafting on the modified surface. Microscope images showed much surface roughness of modified and grafted samples at higher times. These differences could be related to the physical modification or roughness of modified samples with oxygen plasma , also much radical sites at higher times. Cellular investigations with fibroblast cells showed better adhesion, growth, and viability of acrylamide grafted PU, especially acrylamide grafted PU at 180s modification with oxygen plasma. Therefore, the results indicated that cell adhesion increased by acrylamide grafting on the polyurethane surface. These modified surfaces could be used as biomaterials in medical application.
References
- Chu P.k.,Chen J. y. Wang L.P. Huang N., Materials science and Engineerring R 36 143-206(2002) Review paper: Surface Modification for Bioimplants: The Role of Laser Surface Engineering.
- edited by Gunter Oertel with contributions from L. Abele … [et al.].- Munich: Hanser; 1985. “Polyurethane handbook : Chemistry, raw materials, processing, application, properties” New York: Distributed in the USA by Macmillan Pub. C
- S. Sartori, A. Rechichi, G. Vozzi, M. D’Acunto , E. Heine, P. Giusti, G. Ciardelli “Surface modification of a synthetic polyurethane by plasma glow discharge: Preparation and characterization of bioactive monolayers” Reactive & Functional Polymers 68 (2008) 809–821
- A. Parvin, H. Mirzadeh, M. T. Khorasani )2007)” Physicochemical and Biological Evaluation of Plasma-Induced Graft Polymerization of Acrylamide onto Polydimethylsiloxane” Polymeric Biomaterials Department, Iran Polymer and Petrochemical Institute (IPPI), Tehran, Iran.
- M.T. Khorasani, S. MoemenBellah, H. Mirzadeh, B.Sadatnia (2006) “Effect of surface charge and hydrophobicity of polyurethanes and silicone rubbers on L929 cells response” Colloids and Surfaces B: Biointerfaces 51 (2006) 112–119.
- I.-K. Kang, S.-H. Choi, D.-S. Shin, S.C. Yoon 2001 “Surface modification of polyhydroxyalkanoate films and their interaction with human fibroblasts” International Journal of Biological Macromolecules 28 (2001) 205–212
- H. W. Lu, Q. H. Lu, W. T. Chen, H. J. Xu and J. Yin . Cell culturing on nanogrooved polystyrene petri dish induced by ultraviolet laser irradiation . Materials Letters . 2004;58(1-2):29-32.
- Roucoules G, Mathia T, Lanteri P. Hydrophobic mechano chemical treatment of metallic surfaces.Wettability measurements as means of assessing homogeneity. Adv.in Colloids And Interf. Sciences. 2002;97(1-3):177-201.
- Hay K.M., Dragila M.I., Liburdy J. The oretical model for the wetting of a rough surface . J.of Colloid Interf. Science . 2008;325:472–477.
- Kogelschatz U. Dielectric – barrier discharges: their history , discharge physics and industrial applications. Plasma chem. Plasma .2003; 23:1-46.
- Kuzmichev Al . Ion plasma sources based on a microwave oven . instrum exp tech. 1994;37:648-653.
- Asmussen V. electron cyclotron resonance microwave discharge for etching and thin film deposition . j vac sci techno.1989;7:883-889.
- Takahashi C, jin Y, Nishimura K, Matsuo S. anisotropic etching of Si and WSiN using ECR plasma of SF6-CF4 gas mixture . j appl phys.2000;39:3672-3676.







