Sakshi Sharma and K. Aruna*
Department of Microbiology, Wilson College, Mumbai - 400 007, (India).
Abstract
Potential thermophilic extracellular protease producer Bacillus subtilis WIFD5 was isolated from a milk powder sample. Various parameters were studied for optimum protease enzyme activity. A temperature of 550C and pH 9 gave maximum enzyme activity while 5mM PMSF inhibited the enzyme activity thereby classifying it to be thermophilic serine alkaline protease. Enzyme displayed good activity at 0.5% H2O2 indicating it to be bleach stable. Among different solvents and surfactants tested, 20% v/v toluene and 0.5% Tween-20 exhibited the maximum enzyme activity respectively demonstrating detergent stability of the enzyme. Ca2+ and Mg2+ ions enhanced the enzyme activity suggesting a cofactor requirement for the enzyme.Purification of the enzyme was done using ammonium sulphate precipitation method. Application of protease to remove blood stains from cotton fabrics indicated its potential use in detergent formulations.
Keywords
Protease; PMSF; Detergent; Thermophile
Download this article as:| Copy the following to cite this article: Sharma S, Aruna K. Optimization of Protease Activity of Thermophilic Bacillus subtilis WIFD5 Isolated from Milk Powder. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Sharma S, Aruna K. Optimization of Protease Activity of Thermophilic Bacillus subtilis WIFD5 Isolated from Milk Powder. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2306 |
Introduction
Many microorganisms possess elaborate exoenzymes that are concerned with the degradation of macromolecules whose transport into the cell is restricted because of their size1. Proteases are extracellular degradative enzymes, which catalyze hydrolysis of proteins by peptide bond cleavage. They are a single class of enzymes, which occupy a pivotal position with respect to their application in both pharmaceutical and commercial fields. They represent about 60% of the entire industrial enzyme’s sale in the world due to their applications in several industrial sectors like detergent, food, pharmaceuticals, chemicals, leather, silk, paper and pulp industries2. Microorganisms serve as a source of proteases mainly due to their shorter generation time, the ease of bulk production and the ease of genetic and environmental manipulation3. Production of proteolytic enzymes is a common property and can be found in pathogenic as well as nonpathogenic microorganisms. Despite the larger number of protease producing microorganisms, only a few are considered as appropriate producers for commercial exploitation being ‘generally regarded as safe’ (GRAS)4. Many proteases isolated and characterized from thermophilic organisms are unusually structurally stable under high temperature due to the fact that the organisms producing them are adapted to high temperature environments. Detailed structural and functional investigations of these enzymes are of considerable interest for uncovering the molecular strategies responsible for heightened levels of biomolecular thermostability5. Thermophilic proteases are advantageous in some applications where higher processing temperatures can be employed, resulting in faster reaction rates6.
The most detailed studies have been on thermolysin, a zinc metalloprotease isolated from Bacillus thermoproteolyticus, a strain of B.stearothermophilus. Other well-characterized thermophilic extracellular proteases include the B.stearothermophilus neutral proteases, thermomycolin of Malbranchea pulchella var. sulfurea, thermitase of Thermoactinomyces vulgaris and a protease from Streptomyces rectus7. Bacterial proteases are mostly extracellular, easily produced in larger amounts, thermophilic and active at wider pH range, making them suitable for wider industrial application8.
The present study is focused on optimization of temperature and pH for maximum extracellular protease activity and the demonstration of enzyme stability in presence of metal ions, enzyme inhibitors, oxidizing agent (H2O2), solvents and surfactants. It also includes purification of enzyme along with its use as a potential detergent additive.
Materials and methods
Microorganism and culture conditions
Thermophilic potential protease producer Bacillus subtilis WIFD5 was isolated from milk powder sample. Protease production was optimized in growth medium consisting of (g/l): Lactose 5.0, Peptone 10, MgSO4 2.0, KCl 0.5 and K2HPO4 5.0 at pH 9 with agitation (150 rev/min) at 550C for 24 hrs9. Supernatant was collected by centrifugation at 5000 rpm for 25 minutes and used as a crude extracellular enzyme.
Protease assay
The proteolytic activity of the enzyme was determined using casein as a substrate. Casein was dissolved in 20mM Borate NaOH buffer (pH 9). The assay mixture consisted of 2 ml of substrate and 1 ml of enzyme solution. The reaction mixture was incubated at 550C for 30 minutes. After 30 minutes the reaction was terminated by the addition of 5 ml of 5% trichloroacetic acid (TCA). The mixture was then centrifuged at 5000 rpm for 25 minutes to remove the resulting precipitate. One ml of this supernatant was added to 5 ml of Reagent C. Then 0.5 ml of Folin Ciocalteau reagent was added and incubated for 30 minutes in dark. Absorbance was taken at 660 nm10,6. In control tube TCA was added before addition of crude enzyme solution to the substrate. One proteolytic activity (U) was defined as the amount of enzyme that releases 1 µg of tyrosine per minute, under assay conditions11.
Optimization of temperature for maximum enzyme activity
Optimization of the temperature for protease activity was done for different temperatures; 25, 37, 45, 55, 65, 75, 85 and 950C. The substrate 1% casein dissolved in buffer was incubated with enzyme at respective temperatures in water bath for 30 minutes.
Optimization of pH for maximum protease activity
Optimization of the pH for protease activity was done ranging from pH 4 to 12. The substrate 1% casein was dissolved in buffers of different pH’s ranging from 4 to 12. The buffer used were 0.02M Acetate buffer for pH range 4-5, 0.02M Phosphate buffer for pH range 6-8 and 0.02M Borate buffer for pH range 9-12.
Effect of metal ions on protease activity
The effects of various metal ions like Na2+, Mg2+, Hg2+, Zn2+, Ca2+ and Cu2+ on protease activity were studied. The metal ions used were in the form of salts like NaCl, MgSO4, HgCl2 ,ZnSO4, CaSO4, and CuSO4. The crude alkaline protease was pre-incubated with the above mentioned metal ions at 550C for 30 minutes. The final concentration of each metal ion was 10mM at the time of pre-incubation. The residual enzyme activity (%) was measured by standard protease assay.
Effect of inhibitors on protease activity
The effect of various inhibitors such as phenyl methyl sulphonyl fluoride (PMSF), ethylenediaminetetra acetic acid (EDTA), urea and β-mercaptoethanol on protease activity were investigated to further characterize the enzyme. . The crude alkaline protease was pre-incubated with the above mentioned inhibitors at 550C for 30 minutes. The residual enzyme activity was (%) was measured by standard protease assay. The final concentration of each inhibitor was 5mM at the time of pre-incubation
Effect of oxidizing agent (H2O2) on protease activity
The effect of oxidizing agent such as H2O2 at various concentrations of 0.5, 1 and 5% on protease activity was investigated to determine its effect on the activity. Oxidizing agent at three different concentrations was pre-incubated with the enzyme at 550C for 30 minutes and the residual enzyme activity was calculated at all three concentrations of the oxidizing agent.
Effect of solvents on protease activity
The effects of various solvents such as toluene, 2 propanol, isobutanol, ethanol, methanol and acetone on protease activity were investigated. The crude alkaline protease was pre-incubated with the above mentioned solvents at 550C for 30 minutes. The final concentration of solvents was 20% v/v at the time of pre-incubation. The residual enzyme activity was then determined in percentage by standard protease assay.
Effect of surfactants on protease activity
The effect of various surfactants such as SDS, Tween 20, Tween 80 and Triton X-100 each at three different concentrations of 0.5%, 1% and 5% was determined on enzyme activity by incubating with each surfactant at all the three concentrations at 550C for 30 minutes and then determining the residual enzyme activity. Tween 20, Tween 80 and toluene were dissolved in absolute alcohol.
Purification of protease enzyme
The cultured broth obtained from 24 hrs growth of Bacillus subtilis WIFD5 was centrifuged at 5000 rpm for 25 minutes. The cell free supernatant was collected and concentrated to a level of 75% at 00C using ammonium sulphate and the mixture was incubated overnight at 40C. Precipitated protein was then collected by centrifugation at 5000 rpm for 25 minutes; the resulting pellet dissolved in 20mM Borate NaOH buffer, pH 9.0 containing 2mM MgSO4 and dialyzed exhaustively against the same buffer to remove residual ammonium sulphate. The recovered solution was then used as a crude enzyme.
Application as a potential detergent additive
Wash performance of protease was evaluated by subjecting the blood stain removal test from cotton fabrics. Briefly, application of protease as a detergent additive was studied on white cotton cloth which was cut into 4 cm2 pieces and stained with 1 ml of fresh human blood. The stained cotton pieces were allowed to dry overnight. Following overnight drying; the stained pieces were taken in separate flasks. The following sets were prepared and studied:
1) Flask with distilled water (100 ml) + stained cloth.
2) Flask with distilled water (100 ml) + stained cloth + 1 ml surf detergent (7 mg/ml).
3) Flask with distilled water (100 ml) + stained cloth + 1 ml surf detergent (7 mg/ml) + 2 ml crude enzyme.
The above flasks were incubated at 550C for 15 minutes. After incubation, cloth pieces were taken out, rinsed with water and dried. Visual examination of various pieces exhibited the effect of enzyme in stain removal. Untreated cloth pieces stained with blood were taken as control12.
Results and discussion
Optimization of temperature for maximum protease activity
The optimization of temperature for maximum protease activity was determined by incubating the enzyme-substrate complex at various temperatures ranging from 250C- 950C. The enzyme showed maximum activity at 550C and had a relatively broad temperature adaptability ranging from 450C -750C (Fig. 1). The enzyme retained 67% of the maximum activity at 750C. The enzyme can thus be classified as a thermophilic protease. The cell membrane of thermophiles is made of saturated fatty acids. The fatty acid provides a hydrophobic environment for the cell and keeps the cell rigid enough at elevated temperatures One extremely valuable advantage of conducting microbiological processes at elevated temperatures by using such organisms is reducing the risk of contamination by common mesophiles and their wide industrial uses as they are better suited for harsh industrial processes that are carried out at high temperature conditions 13.
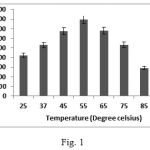 |
Figure 1: Optimization of temperature for maximum protease activity
|
Optimization of pH for maximum protease activity
The enzyme showed maximum activity at pH 9 (Fig. 2) with 20 mM borate-NaOH buffer which makes it useful for commercial detergent solutions as the pH of most commercial detergent solutions is approximately 9. The enzyme retained 70% of its maximum activity between pH 6 to pH 12 indicating its potential for practical use in industrial processes which require activities that cover a broad pH range. This is a very important characteristic for its eventual use in detergent formulations, because the pH of laundry detergents is generally in the range of 9.0–12.014. The ability of B.subtilis WIFD5 to give maximum activity at pH 9 classifies it to be an alkaline protease.
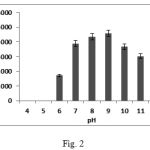 |
Figure 2: Optimization of pH for maximum protease activity
|
Effect of metal ions on protease activity
The effects of some monovalent and divalent metal cations were assessed by measuring casein hydrolysis activity of the enzyme in presence of various metal ions at a final concentration of 10 mM. Ca2+ and Mg2+ ions were found to have a stimulatory effect increasing enzyme activity by up to 127 and 105% respectively (Fig. 3). These results suggest that these metal ions apparently protect the enzyme against thermal denaturation and play a vital role in maintaining active configuration of enzyme at high temperature. It is seen that when interacting with enzymes and other proteins; Mg2+ may bind using inner and outer sphere co-ordination to either alter conformation of enzyme or take part in chemistry of catalytic reaction. Ca2+ may be used as a cofactor to show its full activity. It probably acts as a salt or ion bridge between adjacent amino acid residues very likely via a cluster of carboxylic groups which maintain the rigid conformation of enzyme; a structure related to heat tolerance in thermophilic proteases15.
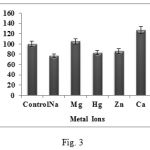 |
Figure 3: Effect of metal ions on protease activity
|
Effect of inhibitors on protease activity
Various enzyme inhibitors at a final concentration of 5mM were used in the study to determine their effects on enzyme activity. It was found that PMSF inhibited the enzyme completely leaving residual enzyme activity to 3%. EDTA, Urea and β-mercaptoethanol gave residual enzyme activity of 82, 87 and 137% respectively which indicated that the enzyme is neither a metalloprotease, nor a cysteine protease (Fig. 4). The residual enzyme activity on pre-incubation with PMSF of only 3% indicates that the B.subtilis WIFD5 enzyme is a serine protease as it is inhibited by PMSF which is a protease inhibitor that inhibits the serine residue in the active site of causing complete loss of activity16. The B. subtilis WIFD5 enzyme under consideration can thus be classified as a thermophilic serine alkaline protease. Inhibitor and activator studies primarily gave insight about the nature of the enzyme, its cofactor requirements and the active centre of the enzyme.
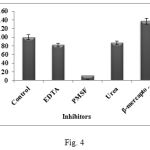 |
Figure 4: Effect of inhibitors on protease activity
|
Effect of oxidizing agent on protease activity
The oxidizing effect of H2O2 was studied on protease activity using three different concentrations of H2O2. After pre-incubation of the enzyme with H2O2 having three different concentrations for 30 minutes, the residual enzyme activity was found to be enhanced with all the three concentrations with 1% H2O2 giving maximum activity (Fig. 5). H2O2 is known to be a strong oxidizing agent and it mediates oxidative inactivation of proteins. Methionine has been identified as a primary site for the oxidative inactivation of the enzyme and most of the subtilisins containing methionine residue next to the catalytic serine residue undergo oxidative inactivation after treatment with oxidizing agents. Therefore the enzyme showing extreme stability towards the oxidizing agents are of immense significance for detergent industries because peroxides are common ingredients of modern bleach based detergent formulations11. Thus as H2O2 was found to stimulate residual enzyme activity by 120.62, 138.91 and 132.62% at concentrations of 0.5, 1 and 5% of H2O2 respectively, the enzyme under study can be classified as bleach stable and hence an excellent candidate for industrial applications as in detergent industry.
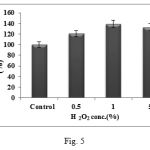 |
Figure 5: Effect of oxidizing agent on protease activity
|
Effect of solvents on protease activity
The effects of different organic solvents (20% v/v) on the enzyme activity were studied on the enzyme under consideration. Several reports suggest that peptide synthesis could be enhanced by addition of organic solvents in the reaction mixture. However enzymes are usually deactivated or denatured in presence of organic solvents. In the present study, following the incubation of enzyme with different solvents; the residual enzyme activity was determined. The enzyme showed stability towards all the solvents including toluene, 2 propanol, isobutanol, ethanol, methanol and acetone giving residual activities of 85, 65, 70, 78, 63 and 75% respectively (Fig. 6). The reasons for comparatively low activity in some solvents could be due to limited dispersion of enzyme, partial denaturation of enzyme and reduced flexibility of proteins in anhydrous solvents. Enzyme activity depends on nature of the organic solvent and maintenance of stereo configuration by it. This means that the replacement of some water molecules in enzyme with organic molecules stabilizes the structure of the enzyme. This may allow Bacillus subtilis WIFD5 protease to be used in organic solvents to shift equilibrium of reversible reaction between hydrolysis and synthesis of peptides to complete the hydrolysis17.
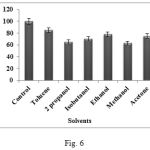 |
Figure 6: Effect of solvents on protease activity
|
Effect of surfactants on enzyme activity
The effects of various surfactants like SDS, Tween-20, Tween-80 and Triton X-100 each at concentrations of 0.5, 1 and 5% was studied on enzyme activity. The enzyme was found to be stable in presence of all the surfactants indicating that the protein may be a well packed structure and native conformation is rigid (Fig. 7). Thermophilic proteases exhibiting activity in alkaline range and high temperature are recognized as potential detergent additives and stain removing formulations. In order to be effective during washing, a good detergent enzyme must be compatible and stable with commonly used detergent compounds such as surfactants, oxidizing agents and other additives that might be present in detergent formulations14. The chemical denaturants and chelators probably affect the native conformation in different ways and increase its conformation flexibility18. The stability of Bacillus subtilis WIFD5 thermophilic enzyme in presence of SDS is particularly important because SDS stable enzymes have rarely been reported. Complete stability of the enzyme under study in presence of various surfactants under varying concentrations suggests its potential in industrial applications.
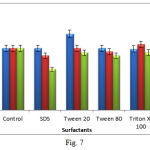 |
Figure 7: Effect of surfactants on protease activity |
Purification of protease enzyme
Purification of the enzyme was obtained at 75% saturation using ammonium sulphate after dialysis which was then used to determine its role as a potential detergent additive (Table. 1). By applying 75% ammonium sulphate precipitation, over 78% of the total protease in the culture filtrate was salted out. Purification achieved with ammonium sulphate was 1.46 fold. This procedure not only facilitated the effective removal of the yellow-colored materials existing in the culture fluid, it also concentrated the enzyme preparation to a workable volume.
Table 1: Purification of Enzyme
| Purification | Total | Total | Specific | Activity | Purification |
| step | Enzyme | protein | activity | % | fold |
| activity (U) | (mg) | (U/mg) | |||
| Culture media supernatant |
2452 |
280 |
8.75 |
100 |
1 |
| Ammonium sulphate precipitation
|
1800 |
140 |
12.85 |
78 |
1.46 |
Application as a potential detergent additive
As the B.Subtilis WIFD5 enzyme showed activity in a broad pH range and broad temperature conditions; with maximum activity at high temperature and compatibility with various surfactants and solvents as well as oxidizing agent; its application as a detergent additive was studied on white cotton pieces stained with human blood (Fig 8 A, B and C). It was found that there was better stain removal from the stained cotton piece that was supplemented with enzyme plus detergent (brand name surf) (Fig. 8 C) than the stained cotton piece that was supplemented with the detergent alone (Fig. 8 B) after incubation at 550C for 15 minutes in a water bath. This provides us with the evidence that the enzyme under consideration has a potential detergent action. The use of proteases in laundry detergents particularly accounts for approximately 25% of the total worldwide sales of enzymes. The detergent proteases work best by hydrolyzing large insoluble proteins. Proteins are initially removed from the fabric surface either by components of detergent matrix or by water alone. Depending upon the size of the resulting fragments, they are either solubilized into the bulk solution, or they deposit themselves back into the fabric. Hence, the best detergent enzyme provides improved substrate hydrolysis, resulting in better stain removal and anti-redeposition benefits14. The obtained result demonstrates that crude enzyme from B.Subtilis WIFD5 can effectively remove stains such as that of blood.
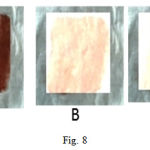 |
Figure 8: Application of protease enzyme as a detergent additive. A) Dried cloth from flask containing D/W, B) Dried cloth from flask containing D/W +1 mg/ml detergent, C) Dried cloth from flask containing D/W + +1 mg/ml detergent + 2 ml enzyme. |
Therefore, B.Subtilis WIFD5 enzyme preparation containing protease activity could be considered as a potential candidate for use as a cleaning additive in detergents to facilitate the release of proteinaceous stains. However, caution must also be exercised in the source of enzymes to be used as detergent additives as some of them might cause allergic reactions in humans19.
Conclusion
The maximum protease activity from thermophilic Bacillus subtilis WIFD5 was found to be at 550C at pH 9 and retained it in presence of other inhibitors except PMSF. It exhibited stability in presence of metal ions, oxidizing agent H2O2 and solvents. In conclusion; it can be classified as thermophilic alkaline serine protease which is bleach stable, active in presence of surfactants and can be used in a number of industrial applications such as detergent industries.
References
- Somkuti AG and Babel JF. Purification and properties of Mucor pusillus Acid protease. 1.J Bacteriol., 1968; 95(4):1407-1414.
- Mukherjee KA, Adhikari H, Rai KS. Production of alkaline protease by a thermophilic Bacillus subtilis under solid-state fermentation (SSF) condition using Imperata cylindrica grass and potato peel as low-cost medium: Characterization and application of enzyme in detergent formulation. Biochemical Engineering Journal, 2007; 39:353-361.
- Ahmed AS, Ramadan A, AI- domany, Nefisa M.A.EI-Shayeb, Hesham H. Radwan and Shireen A Saleh. Optimization, Immobilization of Extracellular Protease and Characterization of its Enzymatic Properties.Research Journal of Agriculture and Biological sciences, 2008;4 (50):434-446.
- Venugopal M and Saramma VA. An alkaline protease from Bacillus circulans BM15, newly isolated from a mangrove station: characterization and application in laundry detergent formulations. Indian J. Microbiol., 2007; 47: 298-303.
- Kocabiyik S and Erdem B. Intracellullar alkaline proteases produced by thermoacidophiles: detection of protease heterogeneity by gelatin zymography and polymerase chain reaction (PCR). Bioresource Technology, 2002; 84:29-33.
- Nascimento WC and Martins ML. Production and Properties of an Extracellular Protease From Thermophilic Bacillus sp. Brazilian Journal Of Microbiology, 2004; 35:91-96.
- Cowan D, Daniel R and Morgan H., Thermophilic proteases: properties and potential applications. Trends in biotechnology, 1985; 3:68-72.
- Banik MR, Prakash M. Laundry detergent compatibility of the alkaline protease from Bacillus cereus. Microbiological Research, 2004; 159:135-140.
- Sharma S, K Aruna. Optimization of protease production by thermophilic Bacillus subtilis WIFD5 isolated from a milk powder sample. Journal of Microbial World, 2011; 13: 241-247.
- Lowry HO, Rosenbrough JN, Farr L, Randall R. Protein measurement with the Folin Phenol Reagent. J Biol Chem., 1951; 265-275.
- Nadeem M, Qazi IJ, Baig S and Syed Q. Effect of Medium Composition on Commercially Important Alkaline Protease Production by Bacillus licheniformis N-2. Food Technol. Biotechnol., 2008; 46 (4):388-394.
- Banerjee CU, Sani KR, Azmi W, Soni R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochemistry, 1999; 35: 213-219.
- Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review. Bioresource Technology, 2003; 89:17-34.
- Hmidet N, Ali N, Haddar A, Kanoun S, Alya S-K, Nasri M. Alkaline proteases and thermosatble α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as a detergent additive. Biochemical Engineering Journal, 2009; 47:71-79.
- Nongporn H-T, Painupong A and Suntinanalert P. Purification and Characterization of an Extracellular Protease from Alkaliphilic and Thermophilic Bacillus sp. PS719. Journal of Bioscience and Bioengineering, 1999; 87(5):581-587.
- Johnvesly B, Naik R.G. Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochemistry, 2001; 37:139-144.
- Wang S, Yang C-H, Liang T-W, Yen Y-H. Optimization of conditions for protease production by Chryseobacterium taeanense TKU001. Bioresource Technology, 2008; 99:3700-3707.
- Zhu W, Cha D, Cheng G, Peng Q, Shen P. Purification and characterization of a thermostable protease from a newly isolated Geobacillus sp. YMTC 1049. Enzyme and Microbial Technology, 2007; 40:1592-1597.
- Anwar A and Salemudin M. Alkaline Proteases:A Review. Bioresource Technology, 1998; 64:175-183.







