Ramya Devi R and Anandhamala G. S.
and Anandhamala G. S.
Department of Computer Science and Engineering, Easwari Engineering College, Chennai, Tamilnadu, India.
Corresponding Author E-mail: ramyadeviresearch@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1533
Abstract
Breast cancer is the leading deadly cancer and most commonly diagnosed in women. New technologies in supplement to existing imaging modalities improve breast cancer screening. This article contributes to identify the high potential device that suggested high accuracy and reliable tool for breast screening and also to examine new screening modalities. An improved imaging system which ensures early detection, non-invasive and radiation free is expected in diagnosis. Numerous imaging modalities like positron emission tomography/computed tomography (PET/CT) imaging, ultrasound, magnetic resonance imaging (MRI), thermography, electrical impedance tomography and few others with recent developments show great potential for diagnosis. Some of the techniques aim for lesion detection and characterization with increased specificity and accuracy. In this paper, the capabilities of traditional and emerging breast imaging modalities used in breast cancer screening are summarized and their advantages and disadvantages are discussed.
Keywords
Breast Cancer; Early Detection; Mammography; Medical Imaging Systems; Thermography
Download this article as:| Copy the following to cite this article: Devi R. R, Anandhamala G. S. Recent Trends in Medical Imaging Modalities and Challenges for Diagnosing Breast Cancer. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Devi R. R, Anandhamala G. S. Recent Trends in Medical Imaging Modalities and Challenges for Diagnosing Breast Cancer. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22895 |
Introduction
The most common type of cancer in women and one of the leading causes of cancer death are breast cancer. According to Information collected from National Cancer Registry Program reports,1 the burden of breast cancer in Indian population is high in 30-60 years age group. It is a rapidly raising rate in younger ages. Risk factors include mainly decreased breast-feeding and physical activity.2,3 Infection may not be felt or detected by existing imaging modality unless a lump becomes sizeable. By the time a lump grows in noticeable size, it usually reaches a minimum of stage 2 cancers.4 So, performing early cancer screening is commendable. Survival rate of the patient will be high if the cancer is detected in the earlier stage.5,6 The early screening protocols include breast awareness, annual screening and clinical breast examination.7 The motive of the article is to identify most economical and effective imaging modality that performs early detection with high accuracy among existing breast screening modalities.
This paper presents a review on recent trends in Medical imaging and challenges. A special attention is given to the study on anatomy of breast and factors that affect breast cancer, since it is the most serious breast pathology.8 Suggesting a best imaging modality in cancer detection is the aim of this review.
This survey is organized as follow. Section 1 gives a brief structure on anatomy of breast and regional lymph nodes which forms vulnerable areas that could be affected by breast cancer. Breast cancer imaging modalities and their potential for diagnosis are discussed in section 2. That includes some pre-screening examinations followed by traditional imaging techniques and emerging trends. Current status in breast cancer imaging is discussed in section 3. Finally, the conclusions of this work are summarized in section 4. The reviewed papers are presented in the bibliography to allow a better understanding in each section.
Breast Anatomy
Breast cancer is malignant tumor that develops in breast cells like milk ducts (ductal carcinoma) or in milk supplying lobules (lobular carcinoma) and spreads into other parts.9 The risk of breast cancer has been associated with mutations in inherited high penetrance genes, age, a family or personal history of breast cancer, reproductive and hormonal factors, hormone replacement therapy (HRT), obesity, alcohol consumption, physical inactivity and exposure to ionizing radiation.10,11 This section briefly describes the anatomy of breast, factors affecting breast cancer and signs, symptoms and efforts for possible treatment for breast cancer.
A woman’s breasts are made up of milk-producing glands. Breast tissue is made up of network of sacs that produce milk termed as lobules and ducts canals.12 Fat covers the lobes and shapes the breast. The female reproductive hormones like oestrogens, progesterone, and prolactin, have a major impact on breast cancer.
![Figure 1: Anatomy of Human breast as illustrated in [12, Fig. 2]](https://biomedpharmajournal.org/wp-content/uploads/2018/09/Vol11No3_Rec_Ram_fig1-150x150.jpg) |
Figure 1: Anatomy of Human breast as illustrated in [12, Fig. 2]
|
Regional Lymph Nodes for Breast
Fig 2 describes regional lymph nodes for Breast. Breasts rests on pectoralis major muscle and attached to the chest wall by ligaments.13 Axillary lymph nodes armpit lymph nodes are located in the underarm to the collarbone above the level of the navel. It includes three clinical classes.14 Class I include underneath the lower edge of the pectoralis minor muscle. Class II includes under the pectoralis minor muscle. Class III is directly above the pectoralis minor muscle. Supraclavicular lymph nodes are present above the collarbone.15,16 The internal mammary nodes located near the breast bone.
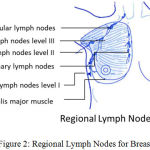 |
Figure 2: Regional Lymph Nodes for Breast.
|
Breast Cancer Imaging Modalities
Breast Imaging – Reporting and Data System (BI-RADS) in 1993 proposed by American College of Radiology has served as guide to standardizing breast imaging reports to improve communication between medical practitioner and patient.17 Such reporting is primarily used in mammogram reporting.18,19 Assessment is divided into seven categories20,21,22
Table 1: BI-RADS assessment category is presented in [17, Tab. 2]
| BI-RADS Categories | Clinical Assessment |
| 0 | Incomplete – no significant abnormality found, Additional imaging modalities required |
| 1 | Negative – no significant distortion found. Like no masses, no calcifications, no asymmetry. |
| 2 | Benign – no malignant lesion found E.g. Cyst, calcification |
| 3 | Probably benign – requires further investigation like biopsy |
| 4 | Suspicious abnormality – requires further investigation, ranges from low suspicious to moderate level |
| 5 | Highly suggestive of malignancy – requires further investigation, more than 95% malignant |
| 6 | Known biopsy-proven malignancy – requires further investigation, breast malignancy already proven |
Diagnosis for breast cancer is a multimodal approach which primarily includes examinations by self and doctor physically and breast screening modalities along with other tests.23 Each imaging modality has significant benefits along with disadvantages.
Pre-Screening Examinations
Clinical-breast examination (CBE) and self-breast examination (SBE) form prescreening processes. Breast screening is performed prior to screening using imaging modalities.24 An individual examining for physical or appearance changes in breast is SBE. Presence of lumps, swelling or distortions may lead for cysts, tumors or other abnormalities.25,26 Detecting breast lesions on regular medical check-up done by a health care provider forms another prescreening procedure in CBE. These two methods hold very less sensitivity. But, they are easy techniques with high specificity.
Table 2: Pre-Screening Examinations
| Modality | Basic Science in Medical Imaging | Application | Sensitivity | Specificity | Advantage | Disadvantage |
| SBE-Self–Breast examination [27] | Detecting breast lesions by looking at and feeling each breast for possible lumps and swelling. | Pre-screening | 12% to 14% | NA | Increases public awareness Easy technique that can be performed at home | Detect early breast cancer
High rates of false positives and over diagnosis |
| CBE-Clinical breast examination [28] | Detecting breast lesions on regular medical check-up done by a health care provider. | Pre-screening | 57.14% | 97.1% | Reduced breast cancer mortality.
Can detect breast cancer missed by mammograph. |
Increased false –positive results.
High rates of false positives and over diagnosis. |
Traditional Imaging Techniques
Mammography
Mammography assesses the anatomical structures of the breast using low-dose x-rays and identifies any abnormalities. The gold standard for diagnosis of breast cancer is mammography since 1960.29 However, sensitivity and specificity are influenced by factors such as breast density, age, stage of infection and family history.30,31 High rate of false –positive mammogram result leads to unnecessary increase in anxiety, worry and increase in stress.
Mammography is not suited for women with dense breasts, fibrocystic breasts and age less than 50. Dense breast tissues and cancer tumor both appear with same property in mammograms, making it difficult to distinguish between two masses.32 As the density of woman’s breast increases the mammography’s ability to detect, abnormalities are reduced. According to American cancer society,33,34 the tissue density of breast was graded into 4 categories. Grade 1 signifies the least dense breast tissue and grade 4 the thickest. Mammogram detection rates were observed as 55% for grade 4, 68% for grade 3 and 83% for grade 2.
During screening process the breast tissues are compressed up to a pressure of 42 pounds. This ruptures the encapsulation of a cancerous tumor cell and release malignant cells into the bloodstream. Other threats of mammography include risk due to radiation. Mammography uses Low dose radiation which increases breast cancer risk.35 Younger women are more susceptible to the effects of radiation than older women because homogeneous cells are more vulnerable to the effects of ionizing radiation. Speculates effect of radiation also leads to BRCA1/2 mutations.36 Women with a family history of breast cancer or BRCA1/2 gene are not preferred to take up mammography.37
PET/CT imaging
Positron emission tomography/computed tomography (PET/CT)38 is the dual scanners that combine classic radiology (CT) and nuclear medicine (PET) imaging in order to merge anatomical and functional details.39 This combination increases the accuracy of images by adding anatomic image registration and localization.40,41 It offers a precise diagnosis, by measuring metabolism with the use of a radiotracer and identifying changes at the cellular level.42,43
Ultrasound
Ultrasound is a diagnosing tool helps to differentiate solid mass from fluid filled masses using high-frequency sound waves. Breast ultrasound has been considered a useful tool in describing abnormality detected in mammograms especially in dense breast. Lesions appear as irregular masses, abnormal enlarged ducts or clustered foci of expanded echogenicity with increased Doppler vascularity.44 Although ultrasound is successfully used to support assessment of abnormalities detected by mammography, it should not be used as a sole modality for screening cancers.45 However, the sensitivity of ultrasound declines in detecting non-palpable tumors such as microcalifications.46,47
Magnetic Resonance Imaging (MRI)
MRI is a non-invasive imaging modality which uses a powerful magnetic field and radio frequency pulses to reproduce detailed pictures of organs, soft tissues and bone.48,49 According to American Cancer Society (ACS) guidelines, MRI is best for diagnosis of breast cancer since it does not involve any harmful radiation for high-risk women with BRCA1 or BRCA2 gene mutation and their first-degree relative.50,51 MRI is more expensive than other imaging modalities. MRI is sensitive to artifacts leading to high false positive results.52 It lacks specificity and identifies a potential lesion with enough specificity only when it is used along with other screening technique.53
Table 3: Traditional Imaging Techniques.
| Modality | Basic Science in Medical Imaging | Application | Sensitivity | Specificity | Advantage | Disadvantage |
| Mammography [34] | Structural Imaging
Using high energy X-rays photon of limited dosage interacts with tissue and gets attenuated. The changes in attenuation is captured and imaged by using reconstruction algorithm |
“Gold Standard”
Prognostic Diagnostic |
68.6% to 83.3% | 90% to 95% | High specificity and sensitivity in detecting cancer.
Portable device Temporal Response (approximately < 1 minute) Good resolution More accuracy in dense breasts when using digital mammography |
False -Positive Prediction cases are high.
Poor contrast compared to CT and MRI. Uses ionizing radiation. |
| PET/CT
Imaging [39] [41] |
Functional Imaging
By annihilation process, High-energy radioactive isotopes create two gamma particles that travel in opposite directions reach towards detectors. Structural Imaging. Beam of X-rays attenuate tissues with interaction. 3D Reconstruction of image is possible. |
Screening, therapeutic diagnostic prognostic | 96% | 77% | Image registration and fusion is accurate. Good contrast Functional information. High sensitivity. | Uses Ionizing radiation. Uses radio isotopes. Poor resolution. Non -portable device Expensive device |
| Ultrasound [46] | Structural imaging Sonoelastograph Acoustic impedance difference | Screening,diagnostictherapeutic | Increases from 36% to 95% with Doppler-3′ | Decreases from 86% to 79% with Doppler” | High diagnostic utility among women with dense breasts” Portable device | High false -positive rates Poor contrast |
| MRI [50] | Structural Imaging
RF pulse deflects protons along transverse plane of applied magnetic field. T1, T2 and T2* relaxation time gives property of tissues. |
Screening, diagnostic prognostic | 88.19% | 67.7% | Nearly Maximum sensitivity [51]
Can detect intra-ductal spread of cancer Good contrast high resolution Nonionizing radiation |
Specificity values are less and variable require compatible equipment.
Biopsies are difficult. Only the lateral side of the breast can be imaged. Not portable Expensive device. |
Emerging Trends
Thermography
A Noninvasive, painless and radiation free imaging modality that help in early detection and risk assessment.54,55 Thermography systems uses infrared camera to produce thermogram images that show patterns of heat and blood flow through thermal emissions on the surface of the body.56 Medical thermography application includes, breast cancer, dentistry, neurology, orthopedics, foot ulcer, pain management, cardiology and veterinary science.57,58 Significance of thermography for breast cancer screening is discussed in detail in the next section.59 Breast thermography was approved by FDA in 1982 as an adjunctive diagnostic breast cancer screening procedure.60
Electrical Impedance Tomography (EIT).
EIT is one of promising emerging technologies that have unique application in imaging; they are in the phase of gaining challenges in clinical and regulatory acceptance.61 Conductivity images produced through EIT are mostly cross-sectional hence it is termed as tomography. [27a] Here, tissues are reproduced by reactive component rather than conductive.62 The electrical properties of malignant tissue of the breast differ prominently to both benign and healthy tissue.63 However, results vary significantly due to different nature of each device and reconstruction approach.64
Microwave Imaging
Microwave excitation was applied to evidence breast cancer.65 Laser infrared thermography with Microwave sources of energy for heating of biological tissues is a part of active dynamic thermal (ADT) imaging.66,67 Unfortunately, due to poor control of microwave energy dissipation, it has limited application. Microwave irradiation generates heat inside a specimen proportional to its dielectric or mechanical properties.68
Optical Imaging
Optical imaging based on geometric optics but is limited to superficial tissue surfaces.69 Optical parameters are quantified at several wavelengths and blood oxygen saturation of tumor and surrounding tissues are estimated.70 Accurate quantification of size and optical properties of breast is a critical requirement for the use of optical imaging.71
Table 4: Emerging Trends in Imaging Techniques.
| Modality | Basic Science in Medical Imaging | Application | Sensitivity | Specificity | Advantage | Disadvantage |
| Thermography [72] | Functional imaging Identifies vascular and temperature changes on skin surface by using emissivity using thermal camera. [73] | Screenin,Diagnostic [74] | 80.5% | 73.3% | Early Detection
Non-invasive Non –radioactive Temporal response Best imaging modality for dense breast. |
Easily affected by room temperature. [75] High false positives and false Negatives. Low Specificity [76] |
| Electrical impedance tomography [77] | Functional and structural Measures local dielectric properties, electrical conductance and capacitance of cancer cells. Scans in approximately | PrognosisScreeningDiagnostic | 72.2% to 38%. | 67% to 95% | Non-invasive Non – radioactive Relatively inexpensive | Poor spatial resolution |
| Microwave imaging [78] | Functional imaging Employs microwave or millimetre waves to image | Diagnostic | Non-invasive Non -radioactive | Poor resolution at higher depth” Low contrast in Fibro glandular tissues | ||
| Optical imaging [79] | Functional imaging Electromagnetic Spectra (650-900nm) use optical fibres. [80] Employs near -infrared light to measure differences in attenuation coefficient and scattering across different tissues. | Screening, diagnostic,
prognostic |
Non-invasive Non –radioactive inexpensive and portable Temporal response Good contrast | High scattering decreases contrast. [81] Limited imaging depth Spatial resolution is less |
Current Status in Breast Cancer Imaging and Discussion
Health care providers recommend mammogram, clinical breast exam, magnetic resonance imaging in women with a high risk of breast cancer. Other screening tests include clinical trials like thermography and tissue sampling
At present, mammography is considered as golden tool of measurement for breast cancer screening. However, mammography does not ensure sufficient screening accuracy with high mammary gland density. Ultrasonography attains better accuracy in breast cancer detection even in dense breasts. Still, the terms for ultrasound equipment and image reading effectiveness have not been standardized. It is commonly used for follow-ups of an abnormality. The role of magnetic resonance imaging (MRI) for breast cancer screening is emerging to simplify features of potential lesion.
Even there is a massive development in the field of screening breast cancer still, clinical breast examination and mammography is recommended with consistent scientific evidences. According to practice bulletin published in July 2017 by American College of Obstetricians and Gynecologists,82 the screening recommendations in precautionary grounds are as given in table 4.
Table 5: Recommendations on breast cancer screening.
| Recommendations | Based on | Screening modality | Study Design |
| Level A | Consistent scientific evidence | clinical breast examination | Beyond screening mammography,
For women aged 25-39 years, for every 1-3 years CBE is considered. Whereas, women aged 40 years and older annual CBE is recommended. |
| mammography Initiation age | Initiate at ages 40-49, Strongly recommended no later than 50 years | ||
| mammography screening interval | Annual or biennial | ||
| mammography stop age | Until age 75 | ||
| Level B | Inconsistent scientific evidence | mammography, ultrasound, MRI and Thermography | Health care providers periodically assess patient’s history. |
| Level C | Expert opinion | self-breast examination, clinical breast examination,mammography and clinical trials like thermography and tissue sampling | Council average-risk women about breast self-awareness and encouraged to notify their health care provider if they experience a change.
Breast self-awareness is defined as a woman’s awareness of the normal appearance and feel of her breasts. |
Cancer screening helps in proper medication if cancer is identified at earlier stages. A combined approach to breast cancer screening increases chances of identifying breast cancer at early treatable stage. But the screening modalities with non-invasive, non-radioactive, inexpensive, portable, with temporal response and good contrast are recommended.
Conclusion
Although Mammography still remains the gold standard for detecting breast cancer, it is still criticized for its effectiveness. There is a need for an imaging modality such that it is free from radiation risk, pain and anxiety, false alarm and non-invasive. Early detection of breast cancer thus necessitates so that treatments are more effective. Ultrasound, Breast MRI and other imaging modalities diagnosis muscle density, fluids and masses. Whereas breast thermography evaluates aberrant thermal emissions on the surface of the body due to increased blood vessel circulation and metabolic changes associated with infection. Since the temperature of cancerous tissues is generally higher than that of healthy tissues, thermograms have been considered a promising screening method for early detection of breast cancer by generating thermograms. PET-CT also plays an important role in staging breast cancer and monitoring treatment response but using ionizing radiations. These imaging modalities used in adjuncts to mammography enhance the ability to detect cancer and assess treatment planning and staging. There is a need for an imaging modality such that it is free from radiation risk, pain and anxiety, false alarm and non-invasive. Early detection of breast cancer thus necessitates so that treatments are more effective and decrease mortality rate.
References
- Malvia S., Bagadi S. A., Dubey U. S and Saxena S. Epidemiology of breast cancer in Indian women. Asia. Pac. J. Clin. Oncol. 2017;1–7.
CrossRef - McPherson K. ABC of breast diseases: Breast can cerepidemiology risk factors and genetics. Bmj. 2000;321(7261):624–628.
CrossRef - Hamajima N., et al. Menarche menopause and breast cancer risk: Individual participant meta-analysis including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151.
CrossRef - Lipari C. a and Head J. F. Advanced infrared image processing for breast cancer risk nassessment. Proc. 19th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. ’Magnificent Milestones Emerg. Oppor. Med. Eng. (Cat. No. 97 CH 36136). 1997;2:673–676.
- Friebel T. M., Domchek S. M and Rebbeck T. R. Modifiers of cancer risk in BRCA 1 and BRCA 2 mutation carriers Systematic review and meta-analysis. J. Natl. Cancer Inst. 2014;106:6.
CrossRef - Zhou Y., Chen J., Li Q., Huang W., Lan H and Jiang H. Association Between Breastfeeding and Breast Cancer Risk: Evidence from a Meta-analysis. Breastfeed. Med. 2015;10(3):175–182.
CrossRef - Gram I. T., Bremnes Y., Ursin G., Maskarinec G., Bjurstam N and Lund E. Percentage density, Wolfe’s and Tabár’s mam mographic patterns agreement and association with risk factors for breast cancer. Breast Cancer Res. 2005;7(5):854.
CrossRef - Mooney G. Breast cancer screening. A study in cost-effectiveness analysis. Soc. Sci. Med. 1982;16:1277–1283.
CrossRef - Ng E. Y. K and Sudharsan N. M. Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer. BMC Cancer. 2004;4:1
CrossRef - Crystal P., Strano S. D., Shcharynski S and Koretz M. J. Using sonography to screen women with mam mographically dense breasts. Am. J. Roentgenol. 2003;181(1):177–182.
CrossRef - Chui J. H., Pokrajac D. D., Maidment A. D. A and Bakic P. R. Towards breast anatomy simulation using GPUs. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) LNCS. 2012;7361:506–513.
CrossRef - Geddes D. T. Inside the Lactating Breast The Latest Anatomy Research. J. Midwifery Women’s Heal. 2007;52(6):556–563.
CrossRef - Rassiwala M et al., Evaluation of digital infra-red thermal imaging as an adjunctive screening method for breast carcinoma: A pilot study. Int. J. Surg. 2014;12(12):1439–1443.
CrossRef - Freedman G. M. et al., Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int. J. Radiat. Oncol. Biol. Phys. 2000;46(4):805–814.
CrossRef - Recht A and Houlihan M. J. Axillary lymph nodes and breast cancer. A review. Cancer. 1995;76(9):1491–1512.
CrossRef - J. J. Albertini et al., Lymphatic Mapping and Sentinel Node Biopsy in the Patient With Breast Cancer. 2015;41.
- Obenauer S., Hermann K. P and Grabbe E. Applications and literature review of the BI-RADS classification. Eur. Radiol. 2005;15(5):1027–1036.
CrossRef - Yoon J. H., Kim M. J., Moon H. J., Kwak J. Y and Kim E. K. Subcategorization of Ultra sono graphic BI-RADS Category 4 Positive Predictive Value and Clinical Factors Affecting It. Ultrasound Med. Biol. 2011;37(5):693–699.
CrossRef - Baker J. A. , Kornguth P. J., Lo J. Y., Williford M. E and Floyd C. E. Breast cancer prediction with artificial neural network based on BI-RADS standardized lexicon. Radiology. 1995;196(3):817–822.
CrossRef - Orel S. G., Kay N., Reynolds C and Sullivan D. C. BI-RADS Categorization As a Predictor of Malignancy. Radiology. 1999;211(3):845–850.
CrossRef - Varas X and Leborgne F. Revisiting the Mam mographic. 2002;691–695.
- Clinic M. 42 / Diagnostic Radiology. 2008;42–43.
- Bezerra L. A. et al., Infrared Imaging for Breast Cancer Detection with Proper Selection of Properties: From Acquisition Protocol to Numerical Simulation. Multi modality Breast Imaging Diagnosis Treat. 2013;227:285–332.
- Ma H., Bernstein L., Pike M. C and Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):43.
CrossRef - Sudharsan N. M., Ng E. Y. K and Teh S. L. Surface Temperature Distribution of a Breast With and Without Tumour. Comput. Methods Biomech. Biomed. Engin. 1999;2(3):187–199.
CrossRef - Feig S. A. et al., Thermography Mammography and Clinical Examination in Breast Cancer Screening: Review of 16,000 Studies. Radiology. 1977;122(1):123–127.
CrossRef - Weiss N. S. in Relation to Clinical Breast Examination and Breast. 2003;9(4):86–89.
- Saslow D. et al., Clinical breast examination practical recommendations for optimizing performance and reporting. CA. Cancer J. Clin. 2004;54(6):327–344.
CrossRef - Friedenson B. Articles Is Mammography Indicated for Women With Defective BRCA Genes ? Implications of Recent Scientific Advances for the Diagnosis Treatment and Prevention of Hereditary Breast Cancer. 2000.
- Fletcher S. W and Elmore J. G. Clinical practice Mammographic Screening for Breast Cancer. N Engl J Med. 2003;348:1672–80.
CrossRef - Berz R and Schulte-Uebbing C. Mammo Vision (Infrared Breast Thermography) Compared to X-Ray Mammography and Ultra sonography 114 Cases Evaluated. Med. Infrared Imaging Princ. Pract. 2012;1–12.
- Bronzino J. D and Peterson D. R. Biomedical Signals Imaging and Informatics. 2014.
- Mettler F. A. r. Essentials of Radiology. 2005;1.
- Shareef M., Ashraf M. A and Sarfraz M. Natural cures for breast cancer treatment. Saudi Pharm. J. 2016;24(3):233–240.
CrossRef - Bird R. E., Wallace T. W and Yankaskas B. C. Analysis of cancers missed at screening mammography. Radiology. 1992;184(3):613–617.
CrossRef - Rimer K., Engstrom P. F and Kessler B. Mammography Barriers. 243–246.
- Jorgensen K. J and Gotzsche P. C. Overdiagnos is in publicly organised mammography screening programmes systematic review of incidence trends. Bmj. 2009;339(1):2587–2587.
CrossRef - Kalles V., Zografos G. C., Provatopoulou X., Koulocheri D and Gounaris A. The current status of positron emission mammography in breast cancer diagnosis. Breast Cancer. 2013;20(2):123–130.
CrossRef - Tsujikawa T., Tsuchida T., Yoshida Y., Kurokawa T and Kimura H. Role of PET CT in Gynecological Tumors Based on the Revised FIGO Staging Classification. 2011;36(9):114–118.
- Sasada S. et al. Which type of breast cancers is undetectable on ring-type dedicated breast PET? Clin. Imaging. 2018;51:186–191.
CrossRef - Lebron-Zapata L and Jochelson M. S. Overview of Breast Cancer Screening and Diagnosis. PET Clin. 2018;13(3):301–323.
CrossRef - Yamamoto Y., Tasaki Y.,Kuwada Y., Ozawa Y and Inoue T. A preliminary report of breast cancer screening by positron emission mammography. Ann. Nucl. Med. 2016;30(2):130–137.
CrossRef - Narayanan D and Berg W. A. Dedicated Breast Gamma Camera Imaging and Breast PET Current Status and Future Directions. PET Clin. 2018;13(3):363–381.
CrossRef - Ben H. P. The Role of Breast Ultrasound in Early Cancer Detection. J. Med. Ultrasound. 2016;24(4):138–141.
CrossRef - Da Y. L et al., Adaptive ultrasound temperature imaging for monitoring radio frequency ablation. PLoS One. 2017;12(8):1–15.
- Huang Q., Zhang F and Li X. Few-shot decision tree for diagnosis of ultrasound breast tumor using BI-RADS features. 2018.
- Yap M. H and Yap C. H. Breast ultrasound lesions classification a performance evaluation between manual delineation and computer segmentation. Proc. SPIE. 2016;9787:978718.
CrossRef - Orel S. G. et al., Staging of suspected breast cancer effect of MR imaging and MR-guided biopsy. Radiology. 1995;196(1);115–22.
CrossRef - Azar F. S., Metaxas D. N and Schnall M. D. A deformable finite element model of the breast for predicting mechanical deformations under external perturbations. Acad. Radiol. 2001;8(10):965–975.
CrossRef - Gubern-Mérida A., Kallenberg M. Mann R. M., Martí R and Karssemeijer N. Breast segmentation and density estimation in breast MRI: A fully automatic framework. IEEE J. Biomed. Heal. Informatics. 2015;19(1):349–357.
CrossRef - O’Flynn E. A. M., Ledger A. E. W and deSouza N. M. Alternative screening for dense breasts: MRI. AJR. Am. J. Roentgenol. 2015;204(2):141–149.
CrossRef - Maxwell A. J., Lim Y. Y., Hurley E.,Evans D. G., Howell A and Gadde S. False-negative MRI breast screening in high-risk women. Clin. Radiol. 2017;72(3):207–216.
CrossRef - de Lange S. V. et al., Reasons for (non)participation in supplemental population-based MRI breast screening for women with extremely dense breasts. Clin. Radiol. 2018;73(8):759.e1-759.e9.
CrossRef - Head J. F., Wang F. E. N and Elliott R. L. PD Flib PLOP PDF Linearization Optimization Protection Page inserted by evaluation version Breast Thermography Is a Noninvasive Prognostic Procedure That Predicts Tumor Growth Rate i n Breast Cancer Patients. Cancer.
- Krawczyk B andSchaefer G. A hybrid classifier committee for analysing asymmetry features in breast thermo grams. Appl. Soft Comput. J. 2014;20:112–118.
CrossRef - González F. J. Theoretical and clinical aspects of the use of thermography in non-invasive medical diagnosis. Biomed. Spectrosc. Imaging. 2017;5(4):347–358.
CrossRef - Amalu F. D. W.C.,DC D. A Review of Breast Thermography. Int. Acad. Clin. Thermol. CA, USA. 2002.
- Am A. J. R and Jan J. R. Current Research Support for Thermography Current Technology Research Supporting Thermography. 2009;180:1.
- Isard H. J., Becker W., Shilo R and Ostrum B. J. Breast thermography after four years and 10,000 studies. Group. 1972;115.
- Acharya U. R., Ng E. Y. K., Tan J. H and Sree S. V. Thermography Based Breast Cancer Detection Using Texture Features and Support Vector Machine. J. Med. Syst. 2012;36(3):1503–1510.
CrossRef - Miguel F.,Luna V., Balleza-ordaz M., Raquel M., Franco H and Riu P. Electrical Impedance Signal Analysis for Medical Diagnosis. 2018.
- Zain N. M and Kanaga K. C. A Review on breast electrical impedance tomography clinical accuracy. ARPN J. Eng. Appl. Sci. 2015;10(15);6230–6234.
- Murphy E. K., Mahara A and Halter R. J. Absolute Reconstructions Using Rotational Electrical Impedance Tomography for Breast Cancer Imaging. IEEE Trans. Med. Imaging, 2017;36(4):892–903.
CrossRef - Bakar A. A., Abbosh A. , Sharpe P and Bialkowski M. Artificial breast phantom for microwave imaging modality. Proc. 2010 IEEE EMBS Conf. Biomed. Eng. Sci. IECBES 2010. 2010;385–388.
- Bicer M. B.,Akdagli A and Ozdemir C. A matching-pursuit based approach for detecting and imaging breast cancer tumor. Prog. Electromagn. Res. M. 2018;64(2017):65–76.
- Dennis W. et al., Prototype System for Microwave Breast Imaging Experimental Results from Tissue Phantoms. 2018;399–402.
- Bahramiabarghouei H., Porter E., Santorelli A., Gosselin B., Popovíc M and Rusch L. A. Flexible 16 antenna array for microwave breast cancer detection. IEEE Trans. Biomed. Eng. 2015;62(10):2516–2525.
CrossRef - Sekkal W., Merad L and Meriah S. M. A Comparative Study for Breast Cancer Detection by Neural Approach for Different Configurations of the Microwave Imaging System. 2018;65:69–78.
- Kamkaew A., Li F., Li Z and Burgess K. An agent for optical imaging of TrkC-expressing breast cancer. Med chemcomm. 2017;8(10):1946–1952.
CrossRef - Zysk A. M. et al., Intraoperative Assessment of Final Margins with a Handheld Optical Imaging Probe During Breast-Conserving Surgery May Reduce the Reoperation Rate: Results of a Multi center Study. Ann. Surg. Oncol. 2015;22(10):3356–3362.
CrossRef - Walsh A. J. et al., Quantitative optical imaging of primary tumor organoid metabolism predicts drug response in breast cancer. Cancer Res. 2014;74(18):5184–5194.
CrossRef - Borchartt T. B., Conci A., Lima R. C. F., Resmini R and Sanchez A. Breast thermography from an image processing viewpoint: A survey. Signal Processing. 2013;93(10):2785–2803.
CrossRef - Gautherie M and Gros C. M. Breast thermography and cancer risk prediction. Cancer. 1980;45(1):51–56.
CrossRef - Rassiwala M. et al., Evaluation of digital infra-red thermal imaging as an adjunctive screening method for breast carcinoma: A pilot study. Int. J. Surg. 2014;12(12):1439–1443.
CrossRef - Moskowitz M. Efficacy of computerized infrared imaging. AJR. Am. J. Roentgenol. 2003;181(2):596. author reply 596.
- Kandlikar S. G. et al. Infrared imaging technology for breast cancer detection – Current status, protocols and new directions. Int. J. Heat Mass Transf. 2017;108:2303–2320.
CrossRef - Mobashsher A. T and Abbosh A. M. Artificial human phantoms: Human proxy in testing microwave apparatuses that have electromagnetic interaction with the human body. IEEE Microw. Mag. 2015;16(6):42–62.
CrossRef - Tajik D., Member S., Foroutan F and Member S. Real-time Microwave Imaging of a Compressed Breast Phantom with Planar Scanning. 2018;7249:1–9.
- Imamura T., Saitou T and Kawakami R. In vivo optical imaging of cancer cell function and tumor micro environment. Cancer Sci. 2018;2017:912–918.
- Jiang L., Zhan W and Loew M. H. A numerical study of the inverse problem of breast infrared thermography modeling. Prog. Biomed. Opt. Imaging – Proc. SPIE. 2010;7626:76260O–76260O–8.
CrossRef - Diot G. et al., Multi spectral Optoacoustic Tomography (MSOT) of human breast cancer. Clin. Cancer Res. 2017;23(22):6912–6922.
CrossRef - Cancer B and Assessment R. Practice Bulletin Number 179 Breast Cancer Risk Assessment and Screening in Average-Risk Women. Obstet. Gynecol. 2017;130(1):e1–e16.
CrossRef







