Manuscript accepted on :13-Sep-2018
Published online on: 17-09-2018
Plagiarism Check: Yes
Reviewed by: Biswajit Batabyal
Second Review by: Jahwarhar Izuan Abd Rashid
Final Approval by: Prof. Juei-Tang Cheng
Ayushi Arora1, Anush Dogra2, Ayush Dogra3 , Bhawna Goyal3
, Bhawna Goyal3 and Apoorav Maulik Sharma3
and Apoorav Maulik Sharma3
1M.B.A pursuing, Punjabi university, Patiala.
2Researcher, Panjab University, Chandigarh.
3UIET,Panjab University, Chandigarh.
Corresponding Author E-mail: ayush12456789@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1479
Abstract
The outbreak of the deadly virus namely nipah virus has been first discovered in Malaysia in 1988. The later outbreaks were recorded in Bangladesh and India. The natural host of this virus is found to be fruit bats. From the fruit bats the virus gets transferred to fruits and vegetables and animals also. Mainly pigs are the ones which easily get infected due to the virus. The fatality rate is very high due to this virus. No vaccine has been yet developed which can cure human infection. In this article the development of nipah virus from 1998 to 2018 is studied and current developments, preventive measures have been studied in order to prevent the future outbreaks.
Keywords
Bioterrorism; Fatal; Infection; Nipah Virus; Paramyxvirus; Vaccine
Download this article as:| Copy the following to cite this article: Arora A, Dogra A, Dogra A, Goyal B, Sharma A. M. Nipah Virus: An Outbreak of Deadly Paramyxvirus. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Arora A, Dogra A, Dogra A, Goyal B, Sharma A. M. Nipah Virus: An Outbreak of Deadly Paramyxvirus. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22594 |
Introduction
In the present era of emerging technology, the act of war between the countries will lead to the complete destruction of the earth because the nuclear weapons which have been developed by the countries for their safety will not only demolish one nation but whole of the world in some or the other way.41 Therefore, any country will not take the risk of using nuclear weapons to destroy their enemy nation. However in order to take revenge now the countries are using biological agents to destroy their competitor country. This act is known as bioterrorism. Bioterrorism attack can be defined as the purposeful release of viruses, bacteria or other biological agents which causes ultimate death and are further transmitted to other organisms.42 The main reason of spreading bioterrorism attack is to affect the productivity of the country which will lead to economic breakdown. The major pathogens which are used as biological weapon are anthrax, plague, equine encephalitis virus etc.40 Recently few years back one new brain damaging virus was discovered which although was a natural phenomenon emergence but many scientists considered it as a biological weapon named as Nipah Virus( NiV).39
It is a form of zoonosis and is a life threatening disease for both animals and human beings. NiV was first discovered in Malaysia in 1999 and was named after the place of discovery which was Sungai Nipah, Malaysia.At that time the hosts were identified as the hosts because the infection was seen in pig farmers.43 However in the later outbreaks no intermediate hosts was seen. The actual identification was first seen in 2001 in Bangladesh. Since then there have been many outbreaks in eastern countries. It had been stated that in Bangladesh the spread was due to the consumption of date palms which had been contaminated with fruit bats.44
In this article we will discussthe outbreak of nipah virus in detail. Following are the sectional details:
The first section will be a detailed study about the various developments of the Nipah Virus.
Second section will deal about the origin of nipah virus in India and the government measures to prevent the outbreak.
The third section will cover the structure of the virus; its epidemiology, structure, causes and symptoms and preventive measures.
The fourth section will include the discussion and the latter will cover conclusion.
The final section will deal about the future prospects related to the development of Nipah Virus.
Review of Literature
A paramyxovirus infection named Nipah infection was distinguished as the cause of narcolepsy in individuals with introduction to the domesticated animals basically pigs in Malaysia and Singapore. The episode was first seen from September 1998 to June 1999. 265 narcolepsy cases, including 105 mortality rates were accounted in Malaysia. 11 instances of respiratory diseases were accounted in Singapore. Electron infinitesimal, serologic, and hereditary examinations demonstrate that this infection has a place with the family Paramyxoviridae and is most firmly identified with the as of late found Hendra infection. The author proposed that these two infections are illustrative of another class inside the breed of Paramyxoviridae. Similar to Hendra infection, Nipah infection is strange to the paramyxoviruses with capacity to contaminate and form possibly deadly ailment in various hosts.1 EphrinB2 is communicated on epithelial cells and neurons which is steady with the known cell tropism for NiV.2 Fundamentally, it concluded that NiV-covered-interceded contamination of epithelial cells and essential rodent nerve cells is restrained by dissolvable EFNB2 gene. Aggregately, it demonstrated that ephrinB2 is a useful receiver for Nipah Virus.3 In 2001 and 2003 ,Two episodes of narcolepsy in places of Meherpur and Naogaon were recorded in Bangladesh, Serum tests were taken from unwell persons, their family units, haphazardly chose occupants, doctor’s facility specialists, and different creatures were obtained. 13 instances (4 affirmed, 9 plausible) in Meherpur; 7 people belonged to the two family units. People who were in close contact with the infected or have contact with debilitated dairy animals were more proved to be more prone to the infection. Pteropus bats were found containing anti toxic bodies against NiV infection. This recommended that transmission can occur by contact with patients or from introduction to a typical origin.4 Nipah infection is an emanate virus that leads to deadly narcolepsy for each penny of contaminated patients5 and the confirmation of person to person transmission is proved.6 Epithelial synctia, contained multi nuclear mammoth cells, are every now and again developed in Nipah diseases, interceded with the combination (F) and connection (G) glycoprotein. The receiver for this infection revealed insight into the path biology of Nipah contamination. EphrinB2, the film headed ligand for the EphB class of receptor tyrosine kinases (RTKs) particularly ties to the connection (G) glycoprotein of NiV.7
The individuals which are able to survive through this battle are fully recovered but 20% cases are still there which are left with lifelong symptoms as personality change or mentally retarded.8 The symptoms in pigs included breathing problems with muscle weakening of age group less tam 1 month old. From 1-6 months symptoms increased to acute fever with cough and nasal discharge. There were also seen neurological symptoms. The infected older animals had aggressive behavior, neurological disorders which ended with sudden death.9 Human infections extendfrom contamination to lethal encephalitis. Tainted individuals at first create flu like side effects of fever, migraine, cough and shortcoming. It could be trailed by hindrance in observation and soundness, unusually tired, modified cognizance, and neurological signs joined by nausea which leads to intensive encephalitis.10
Someindividuals can also posses acute disorders like pneumonia, respiratory diseases which are infected with NiV Bangladesh strain.11 In India, a survey was conducted in different bat populations for the detection of three viruses namely Nipah Virus, Ebola Virus and Marburg Virus. Nearly 140 bats were collected from west Bengal and Maharashtra and the serum samples (IgG) were tested against the prescribed viruses with their RNAs. It was concluded that Nipah Virus Ribo nucleic acid found in the liver of P. giganteus which was from West Bengal. The serum also showed positive result of ELISA for NiV specific IgG. Therefore the origin of NiV inIndia was discovered as the specie of bat namely P.giganteus.12
Past examinations have exhibited hostile to Nipah infection anti toxic body in fruit bats in Indonesia; this investigation gave primary atomic proof that NiV infection for sure courses in populaces of Fruit bats in Indonesia. These discoveries can ideally educate territorial strategy and reinforce developing ailments mindfulness and readiness in Indonesia and area.13 Niv multiplication needs a consistent impart of nucleoprotein (N0) in connection with the phosphoprotein. To clarify the capacity of P, reconstruction was done with nipsh virus. It was concluded that a peptide got from the N-restricting area of P ensures cells against contamination and shown by mutation that peptide hinders N0– P arrangement. The outcomes gave bits of knowledge regarding gathering of N with g RNA and approve the N0– P mind boggling for the use in sedate improvement.14 In spite of this absence of knowledge about NiV infection leads to illness, viral inhibitors are being produced. The monoclonal counter acting agent m102.4 shielded monkeys from Africa to surrender to a generally deadly Nipah virus challenge, notwithstanding under controlled conditions the beginning of infection. Although it is not a full proof research that the antibody can cure the virus in human body still efforts are being conducted on regular basis to create a medical treatment for this deadly virus.15
A study conducted on nipah virus showed that when endothelial cells from brain were used to test the presence of NiVit concluded that increased supply of E selectin leads to the induction of cytokine and it was also figured out that it not only gets increased in the presence of Nipah Virus as a whole but it also be due to the glycoprotein of NiV. This study concluded that it is not necessary to have NiV brain infection in the presence of Nipah virus replication but it can also be due to the presence of NiV glycoprotein.16 The entry of NiV in human central nervous system can be with the help of epithelial cells. In an experiment hendra and nipah virus infection through epithelial cells were studied. It was found that henipaviruses were able to infect the adult sensory nerves. They were also able to replicate easily which resulted in late response of immune system.17 Pteropus.m forms the repository for NiV infection in South Asian countries and gives bits of knowledge about the regular elements, hereditary variety of NiV infection coursing in host. Date palm sap reaping doubtlessly represents the planning and spatial bunching of human Nipah infection encephalitis cases, yet it is unclear when bats seem most drastically averse to shed Nipah infection.18 Avoiding development of new zoonotic infections relies upon understanding determinants for human hazard. In Bangladesh bionomics and huminid social carriers of landformic variety for danger of Nipah virus disease were analyzed. In 2011– 2013, 60 towns instances of disease with Nipah infection were recognized and 147 towns were also taken as control The contrast of case towns and control towns for all probability causes for danger of disease like bats population, people, trees, huninid utilization of date palm sap was conducted. Test towns were like control towns from numerous points of view yet possessed a larger extent of family units who took sap. Lessening person utilization of this can diminish infection transfer and hazard for NiV strain.19 It has been noticed that the virus transmission in Bangladesh among humans is common but when studied the actual reason it has been concluded that people who are associated with already infected person through close contact or are near to their secretions are more infected and the infection from human to human is more common than from bats to humans.20
An antiviral drug known as Favipiravir had been tested against NiV infection in the laboratory conditions. At the highest concentration of the drug used only then the virus replication concentration was found to be decreased a little. It proved that NiV is sensitive to the drug.37
The ongoingdamaging of the common environments of bats has brought about an expanded cover of bat, pets, and human ecologies, which has made open doors for rise of bat-borne zoonotic maladies. Outline of backwoods administration techniques that safeguard bats’ perching and scavenging scenes and avoidance of viral overflow from bats to people require an entire comprehension of the biological story, connecting of bat territory with human and domesticated animals action to clarify when, where, and why an infection develops.38
The following table gives the summary of some of the outstanding work done by scientists on Nipah Virus:-
Table 1: Summary of important discoveries with relation to nipah virus.
| S.No. | Name | Year | Establishment |
| 1. | T.G. Ksiazek et al. | 2000 | Outbreak in Malaysia with 105 deaths |
| 2. | Paul A. Rota et al. | 2000 | Nature of nipah virus |
| 3. | Vincet P. et al. | 2004 | Infection can be from a typical source. |
| 4. | Reynes et al. | 2005 | Paramyxovirusthat leads to encephalitis |
| 5. | Icddr b. et al. | 2005 | Human to human infection is possible. |
| 6. | Poliakov et al. | 2005 | Ephrin b2 ties the receptor and glycoprotein of NiV |
| 7. | Gurley et al. | 2007 | Main host is pteropus genus fruit bats |
| 8. | Hossain et al. | 2008 | Clinical symptoms in pigs |
| 9. | Hughes et al. | 2009 | Human infection can range from no symptoms to lethal flu like symptoms |
| 10. | Wahed et al. | 2011 | Acute diseases like pneumonia can also occur. |
| 11. | Sendow et al. | 2012 | In Asian countries flying fox is the main natural host of the NiV strain. |
| 12. | Yabukarski et al. | 2013 | Peptide along with genomic RNA can be proved to be steady |
| 13. | Geisbert et al. | 2014 | Monoclonal antibody m102.4 invented which proved to be effective against African monkeys |
| 14. | Frietag et al. | 2016 | Brain cells also get activated with the NiVglycoproteins |
| 15. | Borisevich et al. | 2017 | Hepanivirus also were found to be infectious in sensory nerves. |
| 16. | Epstein et al. | 2017 | Reservior for NiV infection in Bangladesh ispteropusmedius. |
| 17. | Gurley et al. | 2017 | Reduced consumption of date palm sap can lead to reduction in the NiV infection cases |
| 18 | Clayton et al. | 2017 | Human to human infection is more common due to the close contact between them |
| 19. | Dawes et al. | 2018 | Favipiravir helps to protect NiV infection in lab conditions |
| 20. | Kumar et al. | 2018 | Environment exploitation is the foremost cause of rehabilitation of infected bats to the domestic areas. |
Nipah Vius: Origin in India
As the studies previously done by various scientists have been discussed above, NiV was first discovered in Malaysia in 1998. At thattime it was recorded that the transmission of the infection was seen in both humans and animals. After the virus outbreak was seen in Bangladesh and it is said that from Bangladesh it had spread to the eastern state of India named West Bengal which appears to be nearest to Bangladesh in 2001. Siliguri town in west Bengal had been reported the site of infection in January 2001. The symptoms of neurological disorders were noticed first with fever. The second outbreak was observed in 2007 in Nadia district. The symptoms were related to respiratory distress and brain related problems were also observed. When the outbreak occurred in 2007 at that point of time it was discovered few bats were hanging near the trees of the NiV infected person which helped to discover that there existed a direct contact between bat and human. Research was conducted and it was found that the transmission of virus in India and Bangladesh was not from pigs to humans but from bats to humans. In India date palm trees had been infected from bat salvia or urine containing NiV which then gets transmitted to humansbyconsuming it as a fruit or from its sap. Recently in 2018 there was the third outbreak in India in the state of Kerala. 17 people had been reported as dead. It was observed that infected patients didn’t seem to transmit the disease which was not in chronic state which prevented the major spread of the disease (20).
Prevention and Control in India
As the scientists are unable to create any permanent cure for the virus transmission and treatment for the same so to prevent is the most important step. The transmission can be refrained by inhibiting the revelation of infected fruit bats and sus to the infection prone areas. The consumption of date palm which are prone to bat excreta or are near to the fruit bats area should also be prevented. A vaccine has been made and used in monkeys to prevent against Hendra and Nipah virus but its use has not been reported against humans.21
Nipah Virus Structure
Epidemiology
Nipah virus has been basically derived from paramyxovirusnipah virus. The genus is Henipahvirus.
It is basically similar to hendra virus. The native reservoir of this virus are basically fruit bats of genus Pteropus genus(27). It has been isolated from the urine of bats. The virus was seen in pigs in Malaysia. Its incubation period in pigs is from 4 to 14 days.28 The pigs can obtain symptoms of respiration problems or diseases related to the neurons. The target site of nipah virus in humans and animals are basically the respiratory tract, brain, kidneys or can also affect Central Nervous System.29
Structure
NiV structure includes a single stranded Ribonucleic acid virus with base pair of 18252. The size ranges from 40-600nm and the shape is pleuromorphic in nature.25 The virus is enveloped in nature. The viral proteins present in the Nipah Virus are fusion protein and attachment glycoprotein. Its polymerase protein helps in the replication and also acts as immunosuppressor.26 The internal structure of nipah virus is shown below:
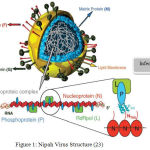 |
Figure 1: Nipah Virus Structure (23)
|
Mode of Transmission
The nipah virus is a virus which is zoonotic in nature which implies it can be transferred from animals to homosapiens and from humans to humans as well easily. The main host of virus is pteronus genus bats or flying fox.24 The humans can get infected by direct contact with the infected animals or through excretions of infected animals. They can also get infected by consuming the fruits or vegetables which were in direct contact with infected animals. Human to human infection happens with direct contact with the infected patient.30 The pathway for infection is shown below:
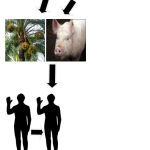 |
Figure 2: Mode of Transmission.
|
Symptoms of Nipah Virus
The various symptoms of the infection includes onset of fever within 5-10 days of the starting infection. Other general symptoms are constant headache, drowsiness, nausea, fatigue, stomach disorders, vision can be disturbed or confusion. The severe symptoms include comma, brain swelling and personality changes.31
Diagnosis of Nipah Virus
NiV can be determined by performing some tests simultaneously. It includes the samples taken from the throat and nasal which are taken for laboratory testing, blood samples, fluid taken from cerebrum and spinal cord and urine tests. The main tests includes RT-PCR, ELISA , virus culture.
Treatment of Nipah Virus
Presently, there is no treatment for the infection against Nipah Virus. Number of vaccines has been made but none of them proved to be effective against humans or animals. The only cure is to detect early symptoms and go for the tests immediately.32 The monoclonal antibody m102.4 had been prepared which successfully identified the NiV G glycoprotein and also proved promising against animals.33
Prevention of Nipah Virus
Though there is no vaccine which can prevent the transmission of infection through Nipah Virus still some measures can be taken in order to prevent it. Farmers who have domesticated animals should prevent their pets from consuming the fruits which are prone to get infected with bats like date palms.34 People should also avoid eating date palms which are near to the bat infected areas. Direct contact of pigs and bats should be avoided. Hospitals should develop proper facilities in order to handle NiV infected patients so that it cannot be further transmitted to other persons. The health care persons should wear gloves and high quality masks.
Discussion
Through this article it can be concluded that nipah virus is proving to be fatal in many Asiancountries, the scientists are continuously working to develop an antiviral against this but till now no successive vaccine has been established which can cure humans against this deadly virus. The transmission of the virus is basically from the fruit bats to animals or trees which ultimately infects the humans. It has also been seen that the infection from human to human is more common if an outbreak occurs compared to animals to humans. Therefore, the countries and states need to take care the after outbreak steps in order to prevent high fatality rate. The infectious persons should be treated carefully in isolation so that people who are in close contact with them do not get infected.
Conclusion
Through this study it has been seen that the outbreaks of Nipah Virus is seen majorly in Asian countries. Its impact on India has also been seen greatly. The fatality rate ranges from 35-65% depending upon the infection severity.45 The death toll due to Nipah Virus in 2018 outbreak in Kerala district of India reached to 17 and the people who were related to the infected patients had been isolated which thereby prevented the spread of disease.46 There is no vaccine or drug developed till now which can cure this fatal disease but the research is still going on. Preventive measures should be taken in the infection prone areas and domesticated animals should be avoided to have direct contact with the fruits which may be infected by fruit bats.47
Future Scope
The upcoming years are probably going to see the progression for the development ofimmunizations against Nipah infection and counteractive action of disease should be studied through changing factors needed for the improvement of therapeutics and treat tainted patients to reduce dismalness and mortality.35 Additionally, there is a probability that NiV may be use as a bio-weapon in the future; hence the probabilities of this should also be taken into consideration. There is also a future question that is there any chance that nipah virus can be more dangerous than HIV which should be studied.36
References
- Chua K. B., Bellini W. J., Rota P. A. , Harcourt B. H., Tamin A. S. K. Lam T. G. K et al., Nipah virus a recently emergent deadly para myxovirus. Science. 2000;288(5470):1432-1435.
CrossRef - Brian H. H., Thomas A. G. K., Pierre E. R., Larry J. A., William J. B and Paul A. R. Molecular characterization of Nipah virus, a newly emergent param yxovirus. Virology. 2000;271(2):334-349.
CrossRef - World Health Organization (WHO). World Organisation for Animal Health (Office International des Épizooties: OIE). Manual on echinococcos is in humans and animals: a public health problem of global concern ( Eckert J. M. A Gemmell F. X. Meslin & Z. S Pawlowski, eds). OIE, Paris. 2001.
- Vincent H. P., Jahangir M. H., Umesh D. P., Monsur M. A., Thomas G. K., Kuzmin I., Niezgoda M., Rupprecht C., Bresee J and Robert F. B. Nipah virus encephalitis reemergence, Bangladesh. Emerging infectious diseases. 2004;10(12);2082.
CrossRef - Jean-Marc R., Counor D., Ong S., Faure C., Seng V., Molia S., Walston J., Georges-Courbot M. C.,Deubel V and Jean-Louis S. Nipah virus in Lyle’s flying foxes Cambodia. Emerging infectious diseases. 2005;11(7);1042.
CrossRef - Icddr B. Person-to-person transmission of Nipah virus during outbreak in Faridpur District, 2004. Health and Science Bulletin. 2004;2(2 ):5-9.
- Alexei P., Cotrina M and David G. W. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Developmental cell. 2004;7(4 ):465-480.
CrossRef - Emily G. S., Joel M. M., Jahangir M. H., Bell M., Azad A., Rafiqul M. I., Abdur M., Molla R., et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerging infectious diseases. 2007;13(7 ):1031.
CrossRef - Hossain M. J., Emily S. G., Joel M. M., Bell M., Darin S. C., Vincent P. H., Formenty P., et al. Clinical presentation of Nipah virus infection in Bangladesh. Clinical Infectious Diseases. 2008;46(7 ):977-984.
CrossRef - James H. M., Mary E. W., Stephen P. L., Emily S. G and Jahangir M. H. Transmission of human infection with Nipah virus. Clinical Infectious Diseases. 2009;49(11)1743-1748.
CrossRef - Farema W., Abdul S. K.,Nessa A and d Mukti M. M. Nipah virus: An emergent deadly paramyxo virus infection in Bangladesh. Journal of Bangladesh Society of Physiologist. 2011;6(2):134-139.
- Pragya Y . D., Chandrashekhar G. R., Anita M. S., Akhilesh C. M., Jonathan S. T., Stuart T. N and Devendra T. M. Detection of Nipah virus RNA in fruit bat (Pteropusgiganteus) from India. The American journal of tropical medicine and hygiene. 2012;87(3):576-578.
CrossRef - Indrawati S., Ratnawati A., Taylor T., Abdul R. M. A., Saepulloh M., Barr J., Wong F., Daniels P and Field H. Nipah virus in the fruit bat Pteropusvampyrus in Sumatera, Indonesia. PLoS One. 2013;8(7): e69544.
CrossRef - Filip Y., Lawrence P., Tarbouriech N., Jean-Marie B., Delaforge E., Jensen M., Ruigrok R. W. H., Blackledge M., Volchkov V and Jamin M. Structure of Nipah virus unassembled nucleo protein in complex with its viral chaperone. Nature Structural and Molecular Biology. 2014;21(9):754.
CrossRef - Thomas G. W., Chad E. M., Joan B. G., Yee-Peng C., Krystle N. A., Feldmann F., Karla A. F., et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Science translational medicine. 2014;6(242): 24282-24282.
CrossRef - Tanja F . C and Maisner A. Early activation of primary brain micro vascular endothelial cells by Nipah virus glycoprotein-containing particles. Journal of virology. 2016;90(5 ):2706-2709.
CrossRef - Viktoriya B., Hakan M. O, Malik B and Rockx B. Hendra and Nipah virus infection in cultured human olfactory epithelial cells. m Sphere. 2017;2(3):e252-17.
- Jonathan E. H. The ecology of Nipah virus and the first identification of a bat pegivirusin’Pteropusmedius’, Bangladesh. PhD diss., Kingston University. 2017.
- Emily G. S., Sonia T. H., Hossain K., Hossain M. S., Sazzad M., Hossain J., Rahman M., Yushuf M. .A. S., et al. Convergence of humans bats trees and culture in Nipah virus transmission Bangladesh. Emerging infectious diseases. 2017;23(9):1446.
CrossRef - Bronwyn C. A. Nipah virus: transmission of a zoo no ticparamyxo virus. Current opinion in virology. 2017;22:97-104.
CrossRef - Mandeep C . S., James A. C., Lowe L., Paul A. R., Pierre E. R., William J. B., Thomas G. K and Akhilesh C. M. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerging infectious diseases. 2006;12(2):235.
CrossRef - Subha G., Kumar V. S., kumar N. P and Kumari N. F. Nipah virus: An update on prevention and control strategies with special reference to the latest outbreak in India. 2018.
- Filip Y.,Lawrence P., Tarbouriech N., Jean-Marie B., Delaforge E., Ringkjøbing M. J., Ruigrok R.W.H., Blackledge M., Volchkov V and Jamin M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nature Structural and Molecular Biology . 2014;21(9):754.
CrossRef - David H. T. S and Johnson N. Nipah Virus: A Virus with Multiple Pathways of Emergence. In The Role of Animals in Emerging Viral Diseases. 2014;293-315.
- Brian H . H., Tamin A., Thomas G. K., Pierre E. R., Larry J. A., William J. B and Paul A. R. Molecular characterization of Nipah virus, a newly emergent paramyxo virus. Virology. 2000;271(2):334-349.
CrossRef - Cynthia G. S., Whistler T., Pierre E. R., Thomas G. K., Paul A. R., William J. B., Daszak P., Wong K. T , Wun-JuShieh and Sherif R. Z. Elucidation of Nipah virus morphogenes is and replication using ultrastructural and molecular approaches. Virus research. 2003;92(1):89-98.
CrossRef - Kay-Sin T.,Tin C. T and Jin K.G. Epidemiological aspects of Nipah virus infection. Neurol J Southeast Asia. 1999;4(1):77-81.
- Khean G. J., Tin C. T., Kong N. C., Seow P. K. T., Kamarulzaman A., Ahmad S. S., Thong K. W., Johan B. J. A., Bing K. C and Kit S. L. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. New England Journal of Medicine. 2000;342(17):1229-1235.
CrossRef - Stephen L. P., Jahangir M. H., Emily S. G., Be-Nazir A., Banu S., Uddin S. K., Homaira N., et al. Recurrent zoo no tic transmission of Nipah virus into humans Bangladesh, 2001–2007. Emerging infectious diseases. 2009;15(8):1229.
CrossRef - Emily G. S., Joel M. M., Jahangir M. H., Bell M., Kalam A. A., Rafiqul M. I., Abdur M.,Molla R .,et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerging infectious diseases. 2007;13(7):1031.
CrossRef - Nusrat H., Rahman M., Jahangir M. H., Jonathan H. E., Sultana R., Khan M. S. U. , Podder G., et al. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiology & Infection. 2010;138(11):1630-1636.
CrossRef - Katharine B . N.,Zhu Z ., Middleton D., Klippel J., Crameri G., Bingham J., Jennifer A. M., et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS pathogens. 2009;5(10):e1000642.
CrossRef - Peter H., Zaki S., Daniels P and Middleton D. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes and infection. 2001;3(4):315-322.
CrossRef - Olivier E., Borisevich V and Rockx B. Pathogenesis of Hendra and Nipah virus infection in humans. The Journal of Infection in Developing Countries. 2013;7(4):308-311.
- Kumar V. M., Verma P., Singh S., Gaur P., Hoda A. S and Pandey S. Nipah Virus-Infectious Agent: An Overview. Int. J. Life. Sci. Scienti. Res. 2018;2455(1716):1716. eISSN.
- Kumar A. K . A and Anoop A..S.K. Deadly Nipah outbreak in Kerala: Lessons learned for the future. Indian Journal of Critical Care Medicine. 2018;22(7):475.
CrossRef - Brian D. E., Kalveram B., Ikegami T., Juelich T., Jennifer K. S., Zhang L., Park A., et al. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Scientific reports. 2018;8(1): 7604.
CrossRef - Kumar A. K . A and Anoop A. S. K. Deadly Nipah outbreak in Kerala Lessons learned for the future. Indian Journal of Critical Care Medicine. 2018;22:7475.
- Rosella F., Illiano E., Paolini F., Massa S., Venuti A and Costantina O. D. Rapid and Low-Cost Tools Derived from Plants to Face Emerging Re-emerging Infectious Diseases and Bioterrorism Agents. In Defence Against Bioterrorism, Springer, Dordrecht. 2018:123-139.
- Preparedness, Bioterrorism. Public health security and bioterrorism preparedness and response act of 2002. Public law. 2002;107(188): 188.
- John J . A., David S. S., David A. A., Omenaca C., Martin S. T., Galbraith M., Tapper M., et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerging infectious diseases. 2001;7(6):933.
CrossRef - Lori H., Thompson W., Matthew G. S and Treadwell T . The bioterrorism preparedness and response early aberration reporting system (EARS). Journal of Urban Health. 2003;80(1):89-96.
- Khean G. J., Tin C. T., Kong N. C., Seow P. K. T., Kamarulzaman A., Ahmad S. S., Thong K. W., Johan B. J. A., Bing K. C and Kit S. L. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. New England Journal of Medicine. 2000;342(17):1229-1235.
CrossRef - Stephen L. P., Rahman M., Jahangir M. H., Lauren S. B., Mushtaq M. H., Gurley E., Khan R .,et al. Food borne transmission of Nipah virus, Bangladesh. Emerging infectious diseases. 2006;12:121888.
- MdZakiul H., Hossain M. S S., Stephen P. L., Sturm-Ramirez K., Uddin M. B., Ziaur M. R., Muzahidul M.d. I .,et al. Nipah Virus Contamination of Hospital Surfaces during Outbreaks, Bangladesh, 2013–2014. Emerging infectious diseases. 2018;24(1):15.
CrossRef - Liz P. Nipah virus in Kerala: A deadly Zoo nos is. Clinical Microbiology and Infection. 2018.
- Rekha M. M. A Short Review on Nipah Virus Infection (NIV). Research & Reviews. A Journal of Immunology. 2018;8(1 ):31-33.







