Manuscript accepted on :28-Apr-2018
Published online on: 12-09-2018
Plagiarism Check: Yes
Reviewed by: Guy-Armel Bounda
Second Review by: Asif Rasheed
Final Approval by: Dr. Ayush Dogra
Meaad F. Sabbah1, Fawzia Alshubali1, Othman A. S. Baothman1,2, Mazin A. Zamzami1, Lobna Shash3, Ibrahim A. Hassan4,5, Aymn T. Abbas6,7 and Mohamed Kamel Abo-Golayel1,8
1Biochemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
2Microbial Toxicology and Natural Products Center, Faculty of Science, King Abdulaziz University, Saudi Arabia.
3Pathology Department, Faculty of Medicine, Ain Shams University, Cairo.
4Faculty of Science, Alexandria University, 21526 El Shatby, Alexandria, Egypt.
5Centre of Excellency in Environmental Studies (CEES), King Abdulaziz University, 80216, Jeddah 21589, Saudi Arabia.
6Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia.
7Biotechnology Research Laboratories, Gastroenterology Surgery Center, Mansoura University, Mansoura, Egypt.
8Medical Research Center, Ain Shams University Hospitals, Ain Shams University, Cairo.
Corresponding Author E-mail: ma.golayel@kau.edu.sa
DOI : https://dx.doi.org/10.13005/bpj/1519
Abstract
Doxorubicin (DOX) is one of the most potent and widely used chemotherapeutic agents to treat several malignancies. However, the clinical use of DOX is seriously restricted due to its acute and chronic cardiotoxic side effects This study investigated the protective effect of (Ajwa) date aqueous extract (AJDAE) against doxorubicin-induced cardiotoxicity in rats. Sixty Wister albino male rats (150-200 gms.) were comprised in our study and divided into six equal groups: group I (untreated control), group II, group III, rats were orally received AJDAE (0.75 & 1.5 gm/ kg.bw) respectively, for 4 weeks, rats of groups IV, V and VI were intraperitoneally injected with one dose of doxorubicin (5 mg/kg.bw) at the end of the 4th week of the study to induce cardiotoxicity, rats of groups V & VI were orally received AJDAE (0.75 & 1.5 gm/ kg.bw) respectively. Cardiac enzymes, lipid profile, SOD, GR, GST, GPx, CAT and MDA in rats’ hearts homogenate, urinary 8OHdG as well as DNA integrity and histopathological changes were investigated in all studied rats.Oral administration of AJDAE (0.75 & 1.5 gm/ kg.bw) attenuated the cardiotoxicity of DOX, improved the cardiac enzymes, lipid profile, reduced the urinary 8OHdG and prohibited the depletion of endogenous antioxidants and suppressed lipid peroxidation (MDA). Moreover, AJDAE enhanced DNA integrity. Histological findings showed that AJDAE (0.75 & 1.5 gm/ kg.bw) administration reduced cardiomyocytes alterations, congestion, edema and the intense cellular stress exerted on myocardial fibers as well as restored the cardiomyocytes architecture. Our data showed that AJDAE obviously resulted in protective effects against DOX-induced cardiotoxicity in rat’s heart. It can be concluded that Ajwa date offers a considerable protection against DOX-induced cardiotoxicity.
Keywords
Ajwa Date; Cardioprotection; DOX
Download this article as:| Copy the following to cite this article: Sabbah M. F, Alshubali F, Baothman O. A. S, Zamzami M. A, Shash L, Hassan I. A, Abbas A. T, Abo-Golayel M. K. Cardioprotective Effect of Ajwa Date Aqueous Extract on Doxorubicin-Induced Toxicity in Rats. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Sabbah M. F, Alshubali F, Baothman O. A. S, Zamzami M. A, Shash L, Hassan I. A, Abbas A. T, Abo-Golayel M. K. Cardioprotective Effect of Ajwa Date Aqueous Extract on Doxorubicin-Induced Toxicity in Rats. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22481 |
Introduction
Doxorubicin is an effective anticancer drug that is extremely potent in treating patients with numerous types of cancers, such as breast, liver, ovarian, lung and various types of solid tumors.1-4 The occurrence of fatal cardiotoxicity in paediatric as well as in adult patients is characterized by an irreversible cardiomyopathy which compromises the clinical utility of DOX and accounts for the major cause of the chemotherapy related morbidity and mortality.5
The potential mechanisms of doxorubicin-induced cardiotoxicity were not accurate enough. A number of researches suggested that free radical is responsible for DOX-induced cardiotoxicity.6 Furthermore, the supplementation of antioxidants has been revealed to protect heart tissue from DOX-induced oxidative damage.
To limit the DOX-induced cardiotoxicity, several molecules such as beta blockers, angiotensin receptor blockers, amifostine, dexrazoxane, Mesna (2-mercaptoethane sulfonate Na), leucovorin, and erythropoietin, have been evaluated as cardioprotective adjuvants in preclinical studies.7 Recently, dexrazoxane, when subjected to clinical trial against combating DOX-induced cardiotoxicity, exhibited marked cardioprotection and did not compromise the anticancer activity of DOX.8
Numerous studies demonstrated that some natural products possess high antioxidant activities, for example fruits and other medicinal plants which might be utilized to prevent or cure some chronic non infectious diseases initiated by oxidative stress.9-11 Phytochemically, the active biocomponents of date palm have drawn great attention within several researchers and clinicians as a result of their lowering of cholesterol, antioxidant activity, prevention of diabetes, chemoprevention of cancer and cardiovascular diseases.12,13 The mechanisms by which restoration of morphology and functions of heart and coronaries involved preserving vascular endothelial activity, regulating lipids metabolism, controlling blood pressure, suppress platelets function, improving ischemia/reperfusion injury, inhibiting thrombosis, decreasing oxidative stress, and diminishing inflammation.13,14 A lyophilized water date fruit extract was found to improve cardiomyogenesis up to about half of the propagation, as well as reversed the suppression of endogenous antioxidants and repressed lipid peroxidation.15 Therefore, the present study was designed to investigate the probable anti oxidative efficacy of date palm (Ajwa) against DOX-induced cardiotoxicity in Wistar albino rats.
Materials and Methods
Animal Models
Sixty male Wistar albino rats (150–200g), 6–8 weeks old, were obtained from the King Fahd Medical Research Centre of King Abdulaziz University, KSA. Rats were housed in an animal care facility under room temperature (25± 1 C˚) with 12h light/dark cycles and were given free access to standard pellet diet and tap water ad labium. Before the treatment, rats were left for 7 days to acclimatize. Rats received human care in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The present research protocol was inspected, revised and allowed by the Scientific Ethical Committee of King Abdulaziz University, S.A.
Doxorubicin
Doxorubicin was purchased from EBEWE Pharma Ges.m.b.H. Nfg.KG, A-4866 Unterach, AUSTRIA.
High performance liquid chromatography (HPLC) of (Ajwa) date aqueous extract (AJDAE) (Phoenix Dactylifera L.)
High performance liquid chromatography (HPLC) of date palm (DP) fruits was conducted according to.16,17 The column used for the analysis was waters symmetry C18 (4.6 × 250 mm 5 μm) with a linear gradient of buffer/methanol (96:4) at a constant flow rate of 1 ml/min with 1800 pressure by using waters pump (1515 isocratic). A UV (2487) detector was employed for the detection of peaks, using two channels simultaneously at a wavelength of 210 nm, a bandwidth of 5 nm and another wavelength of 280 nm.16,17
Preparation of AJDAE, treatment of rats, induction of cardiotoxicity and experimental Design
Flesh fruits (10 g) of date palm (Ajwa) were soaked overnight in 100 ml of distilled water and mixed in a mixer at room temperature (25oC±4) as described by Abdelaziz and Ali.18 The aqueous extracts were filtered and centrifuged at 13,000xg, and 4°C for 5 minutes. The residues were discarded and the supernatant were separated and stored at 4oC until use. AJDAE was prepared daily as fresh as used.19 Rats were divided equally and randomly into 6 groups (10 rats/group) six groups.
Group-1: rats of this group served as negative control and receive corn oil daily throughout the experiment and single intraperitoneal injection of 0.9% saline (vehicle of DOX) at the end of the 4th week of the study.20
Starting from day one of the experiment, rats of group II, group III, rats were orally received 1.5 & 3 ml of AJDAE (0.75 & 1.5 gm/ kg.bw respectively), for 4 weeks using intra-gastric gavage; the volume of AJDAE was increased in accordance with the increase in the rats’ body weight..
Rats of groups V and VI were orally received 1.5 & 3 ml of AJDAE (0.75 & 1.5 gm/ kg.bw respectively) for 4 weeks using intra-gastric gavage; the volume of AJDAE was increased in accordance with the increase in the rats’ body weight.
All rats of groups IV, V and VI were intraperitoneally (I.P) injected with a single dose of DOX (5 mg/kg bw.) at the end of the 4th week of the study to induce myocardial injury.21
Samples Collection
Twenty four hours post DOX injection, the studied rats were anesthetized with pentobarbitone sodium (60 mg/kg–1) and blood sample from each rat was withdrawn from the optic vein, collected in a centrifuge tube and kept at room temperature for 20 minutes. By using cooling centrifuge, sera were separated by centrifuging tubes at 3000 rpm for 15 minutes. Each serum sample were used for estimation of AST, CK-NAC& CK-MB by the optimized standard kinetic method (EGY-CHEM for lab technology, Bader city, Egypt).Troponin-T (TN-T) was measured by double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) to assay the level of Rat Troponin-T in samples (Bioassay Technology Laboratory). Triglycerides, cholesterol and HDL were estimated by direct colorimetric method (EGY-CHEM for lab technology, Bader city, Egypt). Then, the abdomen of each rat was excised; the heart was removed and divided into three portions. One portion was immersed immediately into 10% buffered neutral formaldehyde solution and processed for histopathological examination, the second portion was used for DNA extraction and the third portion of each rat was consumed for preparation of heart tissue homogenate.
Preparation of cardiac tissue homogenates
The homogenization of hearts’ tissues were carried out immediately after excision in a Teflon-glass homogenizer. The tissue samples were kept at 2-8°C in an ice bucket until they thawed. Two hundred mgs of heart tissue from each studied rat were weighted up and immersed in 2 ml of PBS/ 1mM EDTA. The tissue samples were homogenized thoroughly and were kept for one cycle of freezing down in -80oC freezer. The homogenates were centrifuged at 18,000 × g (+4°C) for 30 min. The supernatant of each homogenized tissue sample was collected carefully, divided in Eppendorf tubes and kept at -80oC ready for use.22
Oxidative and antioxidative Markers
The activities of Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), Glutathione-S-Transferase (GST), Glutathione Reductase (GR) Catalase (CAT) and Malonaldehyde (MDA) were estimated in the cardiac tissue homogenates by applying the competitive enzyme immunoassay technique for all the studied rats using Kits from (Bioassay Technology Laboratory, SHANGHAI KORIAN BIOTECH CO.).
Urine collection and labelling for determination of 8-hydroxy-2 deoxyguanosine (8-OHdG) in urine
Sample preparation, clean-up and derivatization
Urine samples were centrifuged at 2000 3 g for 15 mins and the supernatant of each urine sample was collected and stored at – 19 °C. Internal standard (8OHdG) was used (Aldrich, UK).23 The freeze-dried samples were purged with pure nitrogen and sealed with short thread caps with Teflon septa (Andover, MA, U.S.A.). Acetonitrile / ethane thiol (15 μl; 3:1, v/v) was added to the vial to solubilize the urinary residue and subsequently 60 μl of bis (trimethylsilyl) trifluoroacetamide (containing 1% trimethylchlorosilane) was added to the vial for derivatization at room temperature (25°C). Fifty pmol of 8-OHdG was added to the urine sample as the internal standard; 1 vol. of 10% formic acid was mixed with 10 vol. of urine, to acidify urine. Samples were incubated at 4°C for 1 hr. Samples were centrifuged at 10000 rpm for 10 min at 4°C. The supernatant was collected and cleaned up with Waters Oasis, HLB Vac cartridges (60 mg of packing material) which were preconditioned first with 5 ml of methanol and then with 8 ml of 20 mM formic acid (pH≈2.75). The acidified urine samples were diluted with 20 mM formic acid to a final volume of 6 ml, and the diluted urine was loaded on to the preconditioned HLB Vac cartridges. The diluted urine passed through the cartridges at approx. 2 ml min-1 under a vacuum of approximately 5 mmHg. The collected fraction that contains 8-OHdG was transferred into an auto sampler vial (La-Pha-Pack, Langerwehe, Germany), frozen in liquid nitrogen at −80°C for 45 minutes and dried with a vacuum freeze dryer (BioTran, Kangwon, South Korea).24
Rat urine collection
At the end of the 4th week, all rats of the studied groups were housed in metabolic cages fed ad libitum. The morning urine samples were collected and frozen down at- 80°C until analysis.25
GC-MS Analysis
The samples were analysed on a GC-MS (Shimadzu, 2010, Tokyo, Japan), which was equipped with an automatic sampler. Helium was the carrier gas with a flow rate of 1 ml min-1. The derivatized sample (1 μl) was injected into the GC injection port using a split ratio of 6:1. Column temperature was first held at 190°C for 2 min, then increased from 190 to 290°C at the rate of 20°C min-1 and finally maintained at 290°C for two more minutes. SIM (selective ion monitoring; m/z 383, 731 and 643 for 8OHdG) was performed for quantification using the electronionization mode at 70 eV, with the ion source maintained at 230°C.26
Quality Assurance/Quality Control
Samples of 0.0, 10.0, 25.0, 50.0, 75.0 and 100.0 pmol of unlabelled 8OHdG were added to 1 ml of urine. Fifty pmol [18O] 8-OHdG was added to each urine sample as an internal standard. These urine samples were cleaned up and analyzed as described previously. The accuracy study was performed in triplicate.
Genomic DNA extraction of rats’ hearts and gel electrophoresis
In accordance with the Purification protocol of total DNA from each rat’s heart tissues, DNA extraction was processed using QIAGEN, DNeasy, RNeasy, QIAGEN Group); Molecular biology grade agarose gel (2% agarose gel in 1x TAE buffer) was prepared according to Raj Kumar.27 Gel electrophoresis was carried out at 100 V constant potential difference for up to one hour. DNA fragments were visualized by UVI tech. photoducumentation system.
Histopathological Examination
Tissue preparation procedure
Histopathological evaluation was performed on all candidate rats in this study (control and tested groups) for cardiac sections. Cardiac wall was excised from each participating rat once sacrificed. Rats that died spontaneously before planned scarification were excluded. All excised cardiac tissue samples were initially fixed with 10% neutral buffered formalin solution once sacrificed then they were routinely processed, embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin and eosin (H&E).
Hematoxylin and Eosin staining procedure
Sections were heated in a 60°C oven for one hour before staining for fixation of tissue on the slide. Slides were then deparaffinised in xylene (2 changes, 10 minutes each). Slides rehydration was performed by placing them in descending grades of alcohol (absolute ethanol for 5 minutes, 90% ethanol for 5 minutes and 70% ethanol for 5 minutes). Then they were rinsed in distilled water for 2 minutes. Staining with haematoxylin was done for 2 minutes followed by washing in running tap water until the sections were blue. This was followed by staining with eosin for 1 minute. Slides were then dipped in 90% ethanol once, and then transferred to absolute alcohol (2 changes 2 minutes each). Finally, the sections were cleared in 2 changes of xylene (5 minutes each), mounted using Canada balsam and covered with clean glass slide covers.
Evaluation procedure
The pathologist (LSS) examined each tissue section as at least one presentative H and E stained slide through low power examination (x10) for screening as well as higher power magnification for further characterization. All morphological alterations were recorded. Finally, comparative analysis of data was then followed.
A combined qualitative/quantitative score regarding both severity and extent of myocardial fibres changes evaluated cardiac toxicity.28 Assessment of sections of each tested group was compared to Group 1 (control group) for spotting any of the below tabulated microscopic variations.
Characterization of myocardial alterations and their severity were done using high power microscopic examination (x400), while determining the extent of this alteration was implemented using lower power microscopic surveillance (x100). Overall, histologic score was obtained by multiplying the severity and extent score for such changes.
Statistical analysis
Quantitative data were expressed as mean ± SEM. One way analysis of variance (ANOVA) was used for comparing different groups. All analyses were performed using SPSS 18 for Windows (SPSS Inc., Chicago, USA) and differences were considered statistically significant at p<0.05 for all tests.
Results
Sixty Wister albino rats (150-200 gms.) were included in the current study. Rats were divided equally and randomly into 6 groups (n=10 rats/group) as mentioned in materials as methods. Dried DP fruits extract was analyzed for mineral composition using high performance liquid chromatography (HPLC). Results of HPLC for DP fruits revealed the existence of a variety of minerals such as, Vit. B Complex (0.003 mg g-1), Flavonoids (101.76mg g-1), Mg (7.5mg g-1), Mn (80mg g-1), Fe (2.1 mg g-1), Cu (5.3 mg g-1), K (0.01 mg g-1) and Total Phenolic compounds (66.16mg g-1)(Table 1).
Table 1: Analysis of DP fruits extract mineral composition using high performance liquid chromatography HPLC).
| Ingredients of Ajwa fruits | Conc. (mg g-1) |
| Vit. B Complex | 0.003 |
| Flavonoids | 101.76 |
| Mg | 7.5 |
| Mn | 80 |
| Fe | 2.1 |
| Cu | 5.3 |
| K | 0.01 |
| Total Phenolic compounds | 66.16 |
Serum levels of cardiac enzymes and lipid profile of untreated, AJDAE administered, Dox treated rats and AJDAE protected rats
As shown in Table 2a & b, cardiac enzymes and lipid profile of untreated control rats, AJDAE administered rats, Dox treated rats and AJDAE protected rats through the whole study, the mean serum values of AST of 0.75 gm/ kg.bw AJDAE administered rats is significantly (P≤0.01) lower than that of normal control rats. The results also revealed that, the mean serum values of AST and LDH of 1.5 gm/kg.bw AJDAE administered rats are significantly lower (P≤0.05) than that of the untreated rats.
Table 2a: Cardiac enzymes serum levels of untreated, AJDAE administered, DOX treated and AJDAE protected rats.
| Groups | AST (U/L) | CK-NAC (U/L) | CK-MB (U/L) | LDH (U/L) | Troponin T (pg/ml) |
| Negative Control | 80.316± 4.27 | 10.2±1.48 | 151.28±9.26 | 656.38±39.99 | 3.52±0.39 |
| DP(0.75 gm/kg bw) | 58.603±6.57** | 9.2±1.48 | 150.22±21.36 | 631.29±18.51 | 4.07±0.32* |
| DP (1.5 gm/kg bw) | 56.73±7.404** | 9.2±1.303 | 134.72±14.45 | 547.75±12.43* | 3.75±0.39 |
| Dox | 143.26±10.7** | 19.57±1.718** | 257.49±21.49** | 961.34±39.99** | 5.91±0.37** |
| Dox + DP(0.75 gm/kg bw) | 83.808±10.7## | 9.1±1.516## | 135.35±21.14## | 661.21±20.10## | 4.20±0.425## |
| % of Change | – 41 | – 53 | – 47 | – 31 | – 29 |
| Dox + DP (1.5 gm/kg bw) | 58.61±3.86## | 8.5±1.27## | 123.13±10.25## | 566.16±62.7##++ | 3.59±0.37##+ |
| % of Change | -59 | -56 | – 52 | – 41 | – 39 |
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p< 0.05 and p< 0.01 as indicated by (*) & (**) compared to normal control; by (#)& (##)compared to Dox treated group and by (+)&(++) compared to Dox +0.75 gm/kg bw DP treated group, respectively.
The mean serum values of AST, CK-NAC, CK-MB, Triglycerides, cholesterol, LDH and Troponin T of DOX treated rats are significantly higher (P≤0.01) than those of the normal control rats accompanying with significant decrease (P≤0.01) of the mean serum level of HDL of DOX treated rats compared with that of the untreated rats.
 |
Figure 1: Levels of AST, CK-NAC, CK-MB, LDH and Troponin-T in rats treated with date palm (DP) aqueous extract following Dox induced cardiotoxicity as indicated in the figure.
|
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p < 0.05 and p < 0.01 as indicated by (*) & (**) compared to normal control; by (#) & (##) compared to Dox group and by (+) & (++) compared to Dox + 0.75 gm/kg bw DP group, respectively.
As shown in Table 2a&b, the mean serum values of AST, CK-NAC, CK-MB, LDH, Troponin, Triglycerides and Cholesterol of both 0.75 & 1.5 gm/kg.bw AJDAE protected rats are significantly lower (P<0.01) than those of DOX treated rats with (41%, 53%, 47%, 31%, 29%, 37%, 34% & 59%, 56%, 52%, 41%, 39%, 49%, 51% respectively), whereas the mean serum values of HDL of rats of both 0.75 & 1.5 gm/kg.bw AJDAE protected rats are significantly higher (P<0.01) than those of DOX treated rats with (40% & 48% respectively).
Table 2b: Serum levels of lipid profile of untreated, AJDAE administered, DOX treated and AJDAE protected rats.
| Groups | TG (mg/dl) | Cholesterol (mg/dl) | HDL (mg/dl) |
| Negative Control | 27.74±3.78 | 60.081±8.416 | 48.815±3.73 |
| DP(0.75 gm/kg bw) | 27.27±2.91 | 53.06±13.351 | 50.735±4.58 |
| DP (1.5 gm/kg bw) | 26.58±3.63 | 50.77±10.348 | 53.345±5.38 |
| Dox | 39.70±3.428** | 102.67±21.8** | 32.74±2.64** |
| Dox + DP (0.75 gm/kg bw) | 24.82±2.85## | 67.26±8.95## | 45.0±2.86## |
| % of Change | – 37 | -34 | – 40 |
| Dox + DP (1.5 gm/kg bw) | 20.12±2.709##+ | 49.91±11.42##+ | 47.54±2.85## |
| % of Change | – 49 | – 51 | – 48 |
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p< 0.05 and p< 0.01 as indicated by (*) & (**) compared to normal control; by (#) & (##)compared to Dox treated group and by (+) & (++) compared to Dox +0.75 gm/kg bw DP treated group, respectively
Moreover, the present results in Table 2a&b displayed that the mean serum values of AST, LDH, Troponin T, cholesterol, Triglycerides of 1.5 gm/kg.bw AJDAE protected rats are significantly lower (P<0.05) than those of 0.75 gm/kg.bw AJDAE protected rats.
 |
Figure 2: Levels of TG, CHOL and HDL in rats treated with date palm (DP) aqueous extract Dox induced cardiotoxicity as indicated in the figure.
|
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p < 0.05 and p < 0.01 as indicated by (*) & (**) compared to normal control; by (#) & (##) compared to Dox group and by (+) & (++) compared to Dox + 0.75 gm/kg bw DP group, respectively.
Enzymatic activities of antioxidants and oxidant markers in the studied groups
Table 3 shows that, the enzymatic activities of SOD, GPx and GST, CAT in heart homogenate of 1.5 gm/kg.bw AJDAE administered rats are significantly higher (P≤0.05) and (P≤0.01) than those of the normal control rats respectively. Meanwhile, the mean levels of GR, GST and GPX in heart homogenate of DOX treated rats are significantly lower (P≤0.01) than those of the normal control rats accompanying with significant decrease (P≤0.05) in the enzymatic activities of SOD and CAT in heart homogenate of DOX treated rats compared with that of the normal control rats. The mean level of MDA in heart homogenate of DOX treated rats is significantly higher (P≤0.01) than those of the normal control rats.
 |
Figure 3: Levels of SOD, GR, GST, GPx, CAT and MDA in rats treated with date palm (DP) aqueous extract following Dox induced cardiotoxicity as indicated in the figure.
|
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p < 0.05 and p < 0.01 as indicated by (*) & (**) compared to normal control; by (#) & (##) compared to Dox group and by (+) & (++) compared to Dox + 0.75 gm/kg bw DP group, respectively.
Table 3 also shows that, the enzymatic activities of SOD, GR, GST, GPX and CAT in heart homogenate of both 0.75 & 1.5 gm/kg.bw AJDAE protected rats are significantly higher (P≤0.01) than those of DOX treated rats with (53%, 48%, 43%, 34%, 48% & 78 %, 58%, 60%, 46%, 61% respectively), while the mean levels of MDA in heart homogenate of both 0.75 & 1.5 gm/kg.bw AJDAE protected rats are significantly lower (P≤0.01) than those of DOX treated rats with (29% & 39 respectively).
As shown in Table 3, the enzymatic activities of SOD, GPX and CAT in heart homogenate of 1.5 gm/kg.bw AJDAE protected rats are significantly higher (P≤0.01) and (P≤0.05) than those of 0.75 gm/kg.bw AJDAE protected rats respectively. While, the mean level of MDA in heart homogenate of 1.5 gm/kg.bw AJDAE protected rats is significantly lower (P≤0.05) than those of 0.75 gm/kg.bw AJDAE protected rats.
Table 3: Enzymatic activities of antioxidant enzymes and MDA in heart homogenate of untreated, AJDAE administered, DOX treated and AJDAE protected rats.
| Groups | SOD (ng/g tissue) | GR (ng/g tissue) | GST (ng/g tissue) | GPx (ng/g tissue) | CAT (ng/g tissue) | MDA (nmol/ml) |
| Negative Control | 4.23±0.302 | 3.458±0.335 | 4.162±0.231 | 5.598±0.297 | 2.486±0.285 | 3.21±0.34 |
| DP (0.75 gm/kg bw) | 4.16±0.5008 | 3.41±0.152 | 4.212±0.296 | 5.424±0.375 | 2.886±0.264 | 2.786±0.38 |
| DP (1.5gm/kg bw) | 5.162±0.64* | 3.894±0.205 | 4.974±0.465** | 6.192±0.447* | 3.118±0.292** | 2.86±0.236 |
| Dox | 3.842±0.320* | 2.441±0.314** | 2.525±0.253** | 3.655±0.252** | 2.04±0.251* | 4.682±0.227** |
| Dox +DP (0.75 gm/kg bw) | 5.874±0.396## | 3.614±0.152## | 3.612±0.458## | 4.912±0.220## | 3.038±0.160## | 3.314±0.289## |
| % of Change | 53 | 48 | 43 | 34 | 48 | 29 |
| Dox +DP (1.5 gm/kg bw) | 6.863±0.450 ##++ | 3.86±0.123## | 4.03±0.291## | 5.358±0.2208##++ | 3.305±0.144##+ | 2.868±0.234##+ |
| % of Change | 78 | 58 | 60 | 46 | 61 | 39 |
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p< 0.05 and p< 0.01 as indicated by (*) & (**) compared to normal control; by (#)& (##)compared to Dox treated group and by (+) & (++) compared to Dox +0.75 gm/kg bw DP treated group, respectively.
GC-MS analysis of urinary 8OHdG
The present results shows that, the mean concentrations of urinary 8-OHdG of DOX treated rats is significantly higher (P≤0.01) than those of the normal control rats. Also, the mean concentrations of urinary 8-OHdG in both 0.75 & 1.5 gm/kg.bw AJDAE protected rats are significantly lower (P<0.01) than those of DOX-treated rats. Meanwhile, the concentrations of urinary 8-OHdG of 1.5 gm/kg.bw AJDAE protected rats are significantly lower (P<0.01) than those of 0.75 gm/ kg.bw AJDAE protected rats (Figure 4).
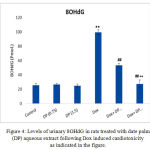 |
Figure 4: Levels of urinary 8OHdG in rats treated with date palm (DP) aqueous extract following Dox induced cardiotoxicity as indicated in the figure.
|
All data were expressed as mean ± SEM. Values were statistically tested using the Student t-test and significant differences at p < 0.05 and p < 0.01 as indicated by (*) & (**) compared to normal control; by (#) & (##) compared to Dox group and by (+) & (++) compared to Dox +DP groups, respectively.
It was establish that the excretion levels (from 101.23 nM to 130.12 nM) of urinary 8OHdG in negative controls were significantly higher than those in treated rats (P < 0.05). The channel shows the ion of m/z 383 (endogenous 8OHdG), and the ion of m/z 385 [18O] 8OHdG as the internal standards). Both endogenous 8OHdG and [18O] 8OHdG had sharp peaks in the chromatogram “the small ones”, well separated from other urinary content peaks. Sharp and isolated peaks of 8OHdG and [18O] 8OHdG could be obtained in most of the urine samples after single SPE.
 |
Figure 5: Typical electrophoretogram of urinary 8OHdG analysis by GC-MS Electrophoretic conditions.
|
Capillary column (length 12 m and internal diameter 0.2 mm) coated with cross-linked 5% phenylmethylsiloxane (film thickness, 0.33 μm; injection: 20 kV×10 s; separation voltage: 20 kV; running buffer: pH 9.12, 30 mM borate buffer; sample matrix: pH 6.5, 30 mM phosphate buffer; detection potential: 0.8V vs. SPE. Peaks represent 8OHdG in negative samples.
DNA Results of untreated, date palm administered and DOX- treated rats and date palm protected rats
DNA was extracted from heart tissues of all the studied rats; the banding manner was obvious in Figure 6. Treatment of rats with DOX increased markedly DNA damage of the cardiac tissues than those of the untreated rats. A totally different banding manner was detected in Dox treated rats which was absent from the heart tissues of the untreated rats. DNA extracted from 0.75 & 1.5 gm/kg.bw of AJDAE protected rat’s heart administered with DOX showed a significant repair in cardiac DNA. Rats treated with only 0.75 & 1.5 gm/kg.bw of AJDAE did not show any sort of DNA fragmentation.
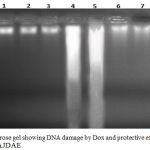 |
Figure 6: Agarose gel showing DNA damage by Dox and protective effects of various fractions of AJDAE.
|
Lanes from left (M) molecular weight marker, (1) Negative control, (2) DP (0.75 gm/ kg. bw.), (3) DP (1.5 gm/ kg. bw.), (4) & (5) Dox , (6) & (7) Dox + DP (0.75 gm/ kg. bw.), (8) & (9) Dox + DP (1.5 gm/ kg. bw.).
Effects of AJDAE on cardiac histoarchitecture
Normal control rats, 0.75 gm/ kg.bw AJDAE administered rats and 1.5 gm/ kg.bw AJDAE administered rats were microscopically unaltered at the LM microscopic examination level except for discrete congested vessels and incidental mild oedematous changes. (Figure 8-A&B). DOX treated cardiac tissue samples revealed the most evident myocardial alterations within all examined groups, none of this group sections scored less than 1.5. Nonetheless, changes did not exceed an overall score of 3 (microvacuolization in widely spread groups of cardiomyocytes). Confluent or extensive myocardial damage was not encountered. Other histopathological alterations in the form of myocardial fibres architectural disarray, as well as cardiomyocytes fragmentation or cytolysis +/- replacement by adipocytes along with congested vessels were also noted, however these findings were not consistently manifested and were not incorporated in the scoring (Figure 8-C & D and Figure 9-A). Groups V and VI cardiac tissue samples (0.75 & 1.5 gm/kg.bw AJDAE protected rats) also expressed a degree of histologic cardiomyocytes alterations. When compared to DOX treated group; these groups (V and VI) displayed milder microscopic alterations of cardiomyocytes in respect to both the extent and severity. On the other hand, these alterations were obvious as compared to normal control group, 0.75 gm/kg.bw AJDAE administered group and 1.5 gm/kg.bw AJDAE administered group. Most of these two groups scored 0.5, maximum score was 2 (Figure 9-B & C). Discrete foci of myocardial fibers fragmentation were occasionally noted in 0.75 gm/kg.bw AJDAE protected rats(Group V). (Figure 9-D) (Table 4, Figure 7).
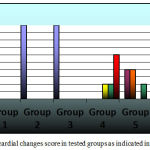 |
Figure 7: Myocardial changes score in tested groups as indicated in key figure.
|
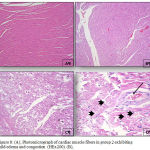 |
Figure 8: (A), Photomicrograph of cardiac muscle fibers in group 2 exhibiting mild edema and congestion (HEx200). (B),
|
Photomicrograph of cardiac muscle fibers in group 2 exhibiting mild edema and congestion (HEx200), (C) Myocardial fibers in group 3 showing architectural disarray and marked fragmentation and cardiomyocytes cytolosis. (HEx200), Figure 4: Photomicrograph of cardiac section in group 3 showing dilated congested vessels (arrow) and cardiomyocytes macro vacuolization (arrow heads) (H&E x400).
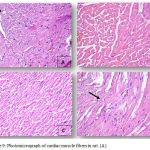 |
Figure 9: Photomicrograph of cardiac muscle fibers in rat. (A).
|
Cardiac section in group 3 showing marked cardiomyocytes fragmentation and cytolysis with replacement by adipocytes (arrow) (H&E x400). (B) Cardiac section in group 4 showing mild architectural disarray, fragmentation and occasional macro vacuolization. (H&E x 400). (C) Section from group 4 showing cardiac myocytes exhibiting mild fragmentation without evidence of myocardiocytes cytolysis. (H&E x200). (D) Myocardial fibers in group 4 section showing mild architectural disarray and rare cardiomyocytolysis (arrow) with replacement by adipocyte. (H&Ex400).
Table 4: Histopathological assessment data of all tested groups.
| Group | Myocardial Damage over all Score | Total | |||||
| 0 No.(%) | 0.5 No.(%) | 1 No.(%) | 1.5 No.(%) | 2 No.(%) | 3 No.(%) | ||
| Group 1 | 10/10 (100%) | – | – | – | – | – | 10 |
| Group 2 | 10/10 (100%) | – | – | – | – | – | 10 |
| Group 3 | 10/10 (100%) | – | – | – | – | – | 10 |
| Group 4 | – | – | – | 2/10(20%) | 2/10(20%) | 6/10 (60%) | 10 |
| Group 5 | – | 4/10 (40%) | 4/10(40%) | – | 2/10 (20%) | – | 10 |
| Group 6 | – | 6/10 (60%) | 2/10 (20%) | 2/10 (20%) | – | – | 10 |
Discussion
This study was conducted to evaluate the possible efficacy of AJDAE in cardio-protection against cardiotoxicity induced by doxorubicin in Wistar albino rats, using biochemical, molecular and histopathological investigations. The present results showed significant decrease in AST serum level in 0.75 &1.5 gm/kg.bw AJDAE administered rats compared with that of the normal control. Furthermore, the present results showed significant decreases in the serum levels of LDH of 1.5 gm/kg.bw AJDAE administered rats respectively compared to that of the normal control. This indicates that there were neither toxic nor hazardous effects of oral administration of 0.75 &1.5 gm/kg.bw AJDAE on the studied rats.
The present work deals with the cardiac toxic side effects of Dox in healthy rats and mainly showed that Dox significantly altered the activities of cardiac enzymes in addition of significant elevation in the mean serum values of triglycerides, cholesterol, LDH and Troponin T with significant decrease in the mean serum values of HDL.
Myocardial enzymes AST, CK-NAC, CK-MB, LDH and Troponin T, are important to evaluate the cardiotoxicity and congestive heart failure.29 In the present study, serum values of these enzymes Troponin T, triglycerides and cholesterol were also found significantly elevated along with significant increase in HDL of DOX treated rats compared with that of the untreated rats, whereas oral administration of AJDAE markedly reversed this elevation, indicating cardioprotective efficacy of AJDAE against DOX cardiotoxicity.
The present results agreed with that of Abba and his colleagues,30 also matched with those of various authors31-34 who reported that the increase in the concentration of enzymes (CK-MB, LDH and AST) led to cardiac toxicity. Abba and his colleagues30 had mentioned that the myocardial necrosis, that often accompanied with the alteration of the heart cell membrane associated with the loss of function of the latter. They demonstrated that ALT, AST, CK-MB and LDH present in myocytes are present in the serum, whereas, their high concentration significantly higher than those of the control group for each studied parameter.30 Therefore, the results of their study confirm the cardiotoxicity of Dox as already stated by other researchers.31,35,36
The results of the current research revealed that the exposure to DOX initiated an intrarenal oxidative stress with significant increase of ROS generation in cardiomyocytes and displayed a significant elevation in the MDA concentration as well as significant reduction in the activities of SOD, GR, GST, GPX and CAT in cardiac tissue of diseased rats. The present results agreed with those of Ashraf and his colleagues37 who demonstrated that DOX treated rats showed significant elevation in the levels of CK-NAC, CPK-MB, LDH, ALT, AST, troponin T and MDA, and significant reduction in the levels of GSH, SOD & CAT. Also the results of the present study were in consistent with that of Amr and his colleagues38 who stated that significant increments of serum troponin T, CK-MB, and MDA levels in cardiac tissue were associated with a significant decrease in GSH in cardiac tissue as a result of DOX administration compared with the untreated control rats. In addition, Hsu and his colleagues proved the involvement of ROS in the generation of myocardial injury and congestive cardiac failure.29
Treatment with AJDAE significantly reduced the elevated values of AST, CK-NAC, CK-MB, Triglycerides, Cholesterol, LDH and Troponin T, and restored them to almost normal values.
The results of the current study also showed that oral administration of AJDAE significantly reduced the increased serum levels of AST, CK-NAC, CPK-MB, LDH, Troponin T, MDA as well as significantly increased the enzymatic activities of SOD, GR, GST, GPX and CAT in cardiac tissue of the AJDAE protected rats of both group 5&6, that have been, orally received 0.75 gm/kg.bw and 1.5 gm/kg.bw respectively, compared with those of the DOX treated group.
The current results also were in consistent with that of Ashraf et al37 who reported that dietary intake of date palm (Ajwa) improved markedly, the enzymatic activities of both cardiac and liver enzymes as well as the antioxidant activities that mirror the cardioprotective efficacy of date palm (Ajwa) against DOX cardiotoxicity.
Rats fed with AJDAE exerted a decreased cardiac tissue MDA levels along with significant improvement in the enzymatic activities of SOD, GR, GST, GPX and CAT that confirms our prediction that date palm (Ajwa) has an antioxidant capacity against DOX-induced cardiomyopathy in rats.
Moreover, the current study revealed that, the mean serum values of AST, triglycerides, cholesterol, LDH and Troponin T of 1.5 gm/kg.bw. AJDAE protected rats displayed a significant decrease compared to that of 0.75 gm/ kg.bw. AJDAE protected rats.
The present results revealed also that the enzymatic activities of SOD, GPX and CAT in cardiac tissue of 1.5 gm/kg.bw AJDAE protected rats were significantly higher than those of 0.75 gm/ kg.bw AJDAE protected rats, accompanied with significant reduction of MDA activity in heart tissue of 1.5 gm/kg.bw AJDAE protected rats compared to those of 0.75 gm/kg.bw AJDAE protected rats.
8-hydroxy-2-deoxyguanosine (8-OHdG) is an stress oxidative biomarker.39 The presence of 8-OHdG is premutagenic and has been known to lead to mutagenesis because it causes G-to-T transversions.38
Cheng and his colleagues40 reported that the concentrations of urinary 8-OHdG were markedly increased in patients with CRC relative to the healthy controls. The noticeable elevation of urinary 8-OHdG may function as a potential non-invasive liquid biomarker for the risk estimation, early warning and detection of CRC.
In spite of being various kinds of DNA oxidative stress products have been identified41 8-hydroxy-2′-deoxyguanosine generated as a result of hydroxyl radical attack of deoxyguanosine residues has been chosen as a biomarker of oxidative stress of DNA.40
The results of the present study demonstrated that rats treated with AJDAE and/or DOX had higher levels of 8-OHdG compared to control group.
There was a significant increment in the excretory levels of urinary 8-OHdG in rats that just received DOX, and decrease in the excretory levels of urinary 8-OHdG from rats following DOX injection and AJDAE mainly with 1.5 gm/kg.bw AJDAE protected rats. This is agreeable to other reports42,43 indicating that increased urinary 8-OHdG excretion after treatment by a cytostatic drug may mirror progressive cellular death and subsequent DNA turnover.44
The association between DOX and 1.5 gm/kg.bw AJDAE was the best treatment as it returned the levels of 8-OHdG to as normal as possible.
One of the expectations of our study was to determine whether doxorubicin induces cardiac DNA injury that was assessed using gel electrophoretic method. The results of the current study showed that DOX induces DNA damage, as presented by a significant increase in the disintegration of DOX treated DNA samples above untreated control in the gel electrophoresis. The present results agreed with that of L’Ecuyer and his colleagues who reported that DNA damage is a primary result in DOX-induced cardiotoxicity through a pathway involving p53 and the mitochondria.45 DNA results revealed that DNA disintegration is a direct result after DOX treatment in rats’ heart tissues that was diminished by inclusion of the free radical scavenger through administration of 0.75 & 1.5 gm/ kg.bw AJDAE. Almost complete restoration of DNA injury was significant after treating rats with 0.75 & 1.5 gm/ kg.bw AJDAE. The protective effect of AJDAE on cardiac DNA injury could be due to the antioxidant activity of AJWA date extract by direct scavenging of ROS or interfering with free radicals generation. A daily intake date palm fruits has been shown to reduce oxidative damage of DNA bases in humans,46 protective for heart disease47 and protect against lipid peroxidation.48 Cardioprotective effects of AJDAE against DOX were also demonstrated on the histological level. Histological evidence proved cardiotoxic effects of DOX on DOX treated rats, 0.75 & 1.5 gm/kg.bw AJDAE protected rats, yet DOX treated rats subjected to DOX administration without subsequent protection by AJDAE administration displayed more striking pathological changes with remarkably higher scores. Like in Al Yahya and his colleagues15 study, DOX cardiotoxicity was manifested as cardiomyocytes vacuolization along with oedema and congestion. Chatterjee and his colleagues also described such changes in their review paper.49 Moreover, separation of cardiac muscle fibres with architectural disarray was also noted, this was also reported by other researchers.50,51 Cardiomyocytes alterations as well as congestion and oedema were assessed in our scoring evaluation; evidently, higher scores were recorded in DOX treated rats as compared to 0.75 & 1.5 gm/kg.bw AJDAE protected rats. Adipocytes permeation amid of the cardiac muscle fibres was frequently encountered in DOX treated rats and only rarely observed in 0.75 & 1.5 gm/kg.bw AJDAE protected rats, this finding was reported to denote metabolic failure of myocardial fibres in case of hypoxia52,53 or alternatively it might reflect metaplastic transformation as an adaptation mechanism in face of cellular stress.54 Both ways, demonstrating such finding reflects an intense cellular stress exerted on myocardial fibres.
All of the above-mentioned alterations were frequently seen in DOX treated rats and much reduced in 0.75 & 1.5 gm/kg.bw AJDAE protected rats, this proves reversibility of DOX cardiotoxicity.
Putting into consideration that the mainstay of our evaluation was gradable in terms of both intensity and extent, such variation in DOX subjected groups can be solidly taken as evidence that AJDAE can ameliorate DOX cardiotoxicity. This outcome is indeed a step forward because it offers an affordable, safe natural product (AJDAE) of evidenced based protective value against hazardous effects of a widely used therapeutic agent (Doxorubicin).
Conclusion
Findings of the current research exhibited that the values of AST, LDH, triglycerides, cholesterol, Troponin T, MDA and urinary 8-OHdG of 1.5 gm/kg.bw AJDAE protected rats were significantly reduced along with significant elevation in the enzymatic activities of SOD, GPX and CAT in heart homogenate of 1.5 gm/kg.bw AJDAE protected rats compared to that of 0.75 gm/kg.bw AJDAE protected rats. This is an indication that 1.5 gm/kg.bw AJDAE accomplished protective and preventive effect on the cardiotoxicity of doxorubicin more than 0.75 gm/kg.bw AJDAE did.
Cancer researchers now commonly accept the benefits of conjoining AJDAE with anticancer drugs. We believe that the combination of doxorubicin and AJDAE can enhance therapeutic properties and diminish the adversative side effects of doxorubicin most of the time. Despite widespread research, the mechanism of doxorubicin-induced cardiac toxicity is still unclear. Further in-vitro and in vivo studies and clinical trials are mandatory for investigating the DOX cardiotoxicity and clarify precisely the mechanism of the antioxidant activities of Ajwa date.
Conflicts of Interest
The authors have no conflict of interests.
Acknowledgements
The authors wish to express a sincere thanks and appreciation to King Abdulaziz City for Science and Technology for its financial support to the research project designated by a number (AI-37-1409).
References
- Clement Y. Can green tea do that? A literature review of the clinical evidence. Prev Med. 2009;49(2-3):83-7.
CrossRef - Dent P. The flip side of doxorubicin Inflammatory and tumor promoting cytokines. Cancer Biol Ther. 2013;14(9):774-5.
CrossRef - Hershman D. L., et al., Doxorubicin cardiac risk factors and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(19):3159-65.
CrossRef - van Dalen E. C., et al., Anthracycline induced cardiotoxicity comparison of recommendations for monitoring cardiac function during therapy in paediatric oncology trials. Eur J Cancer. 2006;42(18):3199-205.
CrossRef - Simunek T., et al., Anthracycline-induced cardio to xicity overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61(1):154-71.
CrossRef - Govender J., et al., Mitochondrial catastrophe during doxorubicin-induced cardio to xicity a review of the protective role of melatonin. J Pineal Res. 2014;57(4):367-80.
CrossRef - Ludke A. R., et al., A concise description of cardioprotective strategies in doxorubicin-induced cardiotoxicity. Can J Physiol Pharmacol. 2009;87(10):756-63.
- Asselin B.L., et al., Cardio protection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol. 2016;34(8):854-62.
CrossRef - Zhou Y., et al., Dietary Natural Products for Prevention and Treatment of Liver Cancer. Nutrients. 2016;8(3):156.
CrossRef - Li A. N., et al., Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020-47.
CrossRef - Li F., et al., Anti proliferative activities of tea and herbal infusions. Food Funct. 2013;4(4):530-8.
CrossRef - Al-Alawi R. A., et al., Date Palm Tree (Phoenix dactylifera L.) Natural Products and Therapeutic Options. Front Plant Sci. 2017;8:845.
CrossRef - Zhao C. N., et al., Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients. 2017;9(6).
CrossRef - Zheng J., et al., Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases. Int J Mol Sci. 2017;18(3).
CrossRef - Al-Yahya M., et al., Ajwa’ dates (Phoenix dactylifera L.) extract ameliorates is oproterenol-induced cardiomyopathy through down regulation of oxidative inflammatory and apoptotic molecules in rodent model. Phy to medicine. 2016;23(11):1240-8.
- Aslam J., Khan S. H and Khan S. A. Quantification of water soluble vitamins in six date palm (Phoenix dactylifera L.) cultivar’s fruits growing in Dubai, United Arab Emirates, through high performance liquid chromatography. Journal of Saudi Chemical Society. 2013;17(1):9-16.
CrossRef - Hamad I., et al., Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules. 2015;20(8):13620-41.
CrossRef - Abdelaziz D. H and Ali S. A. The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepato toxicity in rats. J Ethno pharmacol. 2014;155(1):736-43.
CrossRef - Vayalil P. K. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J Agric Food Chem. 2002;50(3):610-7.
CrossRef - Rashid S., et al., Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol Mech Methods. 2013;23(5):337-45.
CrossRef - Chabner B. A., et al., Antineoplastic agents. In Hardman J. G., Limbird L. E., Gilman A. G. (Eds.) Goodman and Gilman’s the Parmacological Basis of Therapeutics. , ed. Hardman J. G.,Limbird L. E., Gilman A. G. , USA: McGraw-Hill Companies Inc. USA. 2001.
- Yilmaz S., et al. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephro toxicity. Toxicology. 2006;218(2-3):164-71.
CrossRef - Gackowski D., et al., 8-Oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine levels in human urine do not depend on diet. Free Radical Research. 2001;35(6):825-832.
CrossRef - Nakano M., et al., Oxidative DNA damage (8-hydro xydeoxyguanosine) and body iron status a study on 2507 healthy people. Free Radic Biol Med. 2003;35(7):826-32.
CrossRef - Mei S., et al., Determination of urinary 8-hydroxy-2′-deoxyguanosine by two approaches—capillary electrophoresis and GC/MS: An assay for in vivo oxidative DNA damage in cancer patients. Journal of Chromatography B. 2005;827(1):83-87.
CrossRef - Saito S., et al., Quantitative determination of urinary 8-hydroxydeoxyguanosine (8-OH-dg) by using ELISA. Res Commun Mol Pathol Pharmacol. 2000;107(1-2):39-44.
- Gothwal R., et al., Extraction of bulk DNA from Thar Desert soils for optimization of PCR-DGGE based microbial community analysis. 2007;10.
- Manno R. A., et al., The minipig as a new model for the evaluation of doxorubicin-induced chronic toxicity. J Appl Toxicol. 2016;36(8):1060-72.
CrossRef - Hsu H. C., Chen C. Y and Chen M. F. N-3 polyunsaturated fatty acids decrease levels of doxorubicin-induced reactive oxygen species in cardiomyocytes — involvement of uncoupling protein UCP2. J Biomed Sci. 2014;21:101.
CrossRef - Pacôme A. O., et al., Cardioprotective and anti-inflammatory activities of polyphenols enriched extract of Hibiscus sabdariffa petal extracts in wistar rats. Journal of Pharmacognosy and Phytochemistry. 2015;4(1):57-63.
- Andreadou I., et al., Acute doxorubicin cardio toxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol. 2007;42(3):549-58.
CrossRef - Zanwar A. A., Hegde M. V and Bodhankar S. L. Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in is oprenalin induced myocardial necrosis in rats. Interdisciplinary Toxicology. 2011;4(2):90-97.
CrossRef - Radhika J., et al., Cardioprotective role of Justicia Traquebareinsis Linn. Leaf extract in isoproterenol induced myocardial infarction in albino rats. 2013;3:124-128.
- Lalitha G., et al., Protective effect of neferine against isoproterenol-induced cardiac toxicity. Cardiovasc Toxicol. 2013;13(2):168-79.
CrossRef - Abdelbaky N., Ali A and Raeesa M. Cardioprotective effect of simvastatin on doxorubicininduced oxidative cardio toxicity in rats. 2010;629-38.
- Firoz M., et al., Cardioprotective activity of ethanolic extract of Callistemon lanceolatus leaves on doxorubic in-induced cardiomyopathy in rats. 2011;6.
- Abdalla A., et al. Ameliorative Influence of Dietary Dates on Doxorubicin-Induced Cardiac Toxicity. 2016;07:343-353.
- Fouad A. A., et al. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ Toxicol Pharmacol. 2013;36(2):347-57.
CrossRef - Jagetia G andPonemone V. An Indigenous Plant Bael (Aegle Marmelos (L.) Correa) Extract Protects Against the Doxorubicin-Induced Cardio toxicity in Mice. 2015;04.
- Guo C., et al., Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-deoxyguanosine by UPLC-MS/MS Analysis. Scientific Reports. 2016;6:32581.
CrossRef - Dizdaroglu M and Jaruga P. Mechanisms of free radical-induced damage to DNA. Free Radic Res. 2012;46(4):382-419.
CrossRef - Mei S., et al. Determination of urinary 8-hydroxy-2′-deoxyguanosine by two approaches – Capillary electrophores is and GC/MS An assay for in vivo oxidative DNA damage in cancer patients. 2005;827.83-7.
- Yang Y., et al., Determinants of urinary 8-hydroxy-2′-deoxyguanosine in Chinese children with acute leukemia. Environ Toxicol. 2009;24(5):446-52.
CrossRef - Honda M., et al., Correlation of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a biomarker of oxidative DNA damage, and clinical features of hematological disorders: a pilot study. Leuk Res. 2000;24(6):461-8.
CrossRef - L’Ecuyer T., et al., DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am J Physiol Heart Circ Physiol. 2006;291(3):1273-80.
CrossRef - Kotepui M. Diet and risk of breast cancer. Contemp Oncol (Pozn). 2016;20(1):13-9.
CrossRef - Harasym J and Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30(5):511-7.
CrossRef - Basu S., et al., Lipid peroxidation, DNA damage and total antioxidant status in neonatal hyperbilirubinemia. J Perinatol. 2014;34(7):519-23.
CrossRef - Chatterjee K., et al., Doxorubicin Cardiomyopathy. Cardiology. 2010;115(2):155-162.
CrossRef - Imbaby S., et al., Cardioprotective effects of curcumin and nebivolol against doxorubicin-induced cardiac toxicity in rats. Hum Exp Toxicol. 2014;33(8):800-13.
CrossRef - Salouege, I., et al., Means of evaluation and protection from doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. J Cancer Res Ther. 2014;10(2):274-8.
CrossRef - Bilheimer D.W., et al., Fatty acid accumulation and abnormal lipid deposition in peripheral and border zones of experimental myocardial infarcts. J Nucl Med. 197819(3):276-83.
- Rosnowski A., Ruzyllo W and Chrzanowski M. Ultrastructural aspects of subendocardial fat cells accumulation. Zentralbl Allg Pathol. 1986;131(4):349-55.
- d’Amati G., et al., Myocyte transdifferentiation a possible pathogenetic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch Pathol Lab Med. 2000;124(2):287-90.







