Cardiff Metropolitan University, Llandaff Campus, 200 Western Ave, Cardiff CF5 2YB, United Kingdom.
Corresponding Author E-mail: Beatrice.Ondondo@wales.nhs.uk
DOI : https://dx.doi.org/10.13005/bpj/1478
Abstract
Major obstetric haemorrhage (MOH) is a leading cause of maternal death and morbidity, with the majority of deaths occurring within four hours of delivery. Therefore, prompt identification of women at risk of MOH is crucial for the rapid assessment and management of blood loss to urgently restore haemodynamic stability. Furthermore, as the rate of blood loss during MOH can increase rapidly in the time when laboratory results are awaited, the management of MOH could benefit from point-of-care coagulation testing by the ROTEM analyser which has a quicker turnaround time compared to standard laboratory coagulation tests. A number of studies indicate that ROTEM-based management of MOH has resulted in a significant reduction in massive transfusions and decreased transfusion of concentrated red cells (CRC) and fresh frozen plasma (FFP) due to a reduction in total blood loss. Several reports which have linked MOH to the depletion of fibrinogen reserves indicate that the reduction in CRC and FFP transfusions is largely due to an increase in early fibrinogen replacement therapy which corrects hypofibrinogenemia. This short report discusses preliminary findings on the impact of ROTEM point-of-care haemostasis analyser on the transfusion of various blood products to obstetric women experiencing MOH at the Royal Gwent Hospital in South wales. The number of blood products transfused following decisions based on the ROTEM analyser measurements (ROTEM group) was compared to historical transfusion data before the ROTEM analyser became available (Pre-ROTEM group). Blood product transfusion in the Pre-ROTEM group was guided by measurements of standard laboratory coagulation tests in conjunction with the established major haemorrhage protocols at the time. The findings indicate that the ROTEM analyser was effective in managing MOH at point-of-care and led to a reduction in the transfusion of CRC, FFP and platelets. However, contrary to published studies, the reduction in blood product usage was not accompanied by an increase in fibrinogen replacement transfusion therapy, suggesting that the ROTEM’s FIBTEM assay accurately quantified fibrinogen levels based on fibrin-clot firmness to enable an early diagnosis of hypofibrinogenemia. Early establishment of the absence of hypofibrinogenemia helped to prevent unnecessary transfusion of fibrinogen concentrate in this study. These findings support the adoption of routine use of ROTEM analysers at point-of-care on labour wards to manage MOH and reduce fibrinogen replacement therapy. The ease of use and rapidity of ROTEM tests could enable departure from globally directed correction of coagulopathy during MOH to a more focussed and precise target transfusion therapy, which will ultimately reduce blood product wastage (including fibrinogen concentrate) whilst minimising transfusion-associated side effects such as alloimmunisation, circulatory overload and dilutional coagulopathy.
Keywords
Fibrinogen Concentrate; Haemostasis; Major Obstetric Haemorrhage; Point-of-Care; ROTEM; Transfusion Therapy
Download this article as:| Copy the following to cite this article: Ondondo B. O. Management of Major Obstetric Haemorrhage Using ROTEM Point-of-Care Haemostasis Analysers Can Reduce Blood Product Usage Without Increasing Fibrinogen Replacement Therapy. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Ondondo B. O. Management of Major Obstetric Haemorrhage Using ROTEM Point-of-Care Haemostasis Analysers Can Reduce Blood Product Usage Without Increasing Fibrinogen Replacement Therapy. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22437 |
Introduction
Hypofibrinogenemia and its Effects on Obstetric Haemorrhage
Pregnancy and childbirth are associated with decreased levels of fibrinogen.1-3 Moreover, fibrinogen is usually the first factor to fall below critical level during bleeding and haemodilution,4 and in particular during post-partum haemorrhage.5,6 A low level of fibrinogen is associated with poor fibrin-clot quality because fibrinogen is one of the major components of the coagulation cascade, without which the insoluble fibrin strands and a stable blood clot to stop bleeding cannot be formed.7,8 A poor clot quality resulting from low fibrinogen levels correlates with major obstetric haemorrhage (MOH), and a rapid progression to severe post-partum haemorrhage.5,9
A significant association of obstetric haemorrhage with both hypofibrinogenemia and hyperfibrinolysis has been documented, and the level of fibrinogen during and after delivery is used as an early and rapid marker for progression of obstetric haemorrhage.10 Furthermore, fibrinogen level correlates inversely with blood loss6 making it a more sensitive determinant of transfusion volumes used in treating MOH.11,12 Therefore, normalisation of fibrinogen levels by administering fibrinogen concentrate during MOH is thought to be the most effective transfusion therapy to manage both dilutional and consumptive coagulopathy, as it promptly corrects hypofibrinogenemia,13,14 significantly reduces blood loss6,15 and the need for massive transfusions of CRC, FFP and platelets.14-18 However, measurement of fibrinogen concentration by standard laboratory tests may take 60-90 minutes before results are available,19 which delays goal-directed therapy and in urgent cases often leads to empiric treatment for suspected hypofibrinogenemia. Such empiric treatment can result in the unnecessary transfusion of fibrinogen concentrate and other blood products.
Comparatively, the point-of-care ROTEM analyser which gives an accurate assessment of the quality of a fibrin-clot through the FIBTEM assay provides a result within 10 minutes, and can facilitate goal-directed transfusion therapy. Apart from quick turnaround time, the ROTEM’s FIBTEM assay can also identify hypofibrinogenemia, hypofibrinogenesis, and hyperfibrinolysis, all of which are defects found in MOH.6,20 Thus, by establishing the correct level of fibrinogen, the ROTEM analyser can either facilitate early initiation of fibrinogen replacement therapy in case of hypofibrinogenemia or mitigate unnecessary transfusion of fibrinogen to obstetric women with normal or adequate fibrinogen levels. The ROTEM’s FIBTEM-A5 values show a significant positive correlation with fibrinogen concentration, and accurately predict blood loss5 and the likelihood of progression of bleeding to severe obstetric haemorrhage of >2500 ml blood loss.10 Such severe blood loss during MOH can result in hypohaemoglobinemia, thrombocytopenia and depletion of other coagulation factors, necessitating replacement transfusion of CRC, platelets and FFP to restore haemodynamic stability. However, several studies indicate that ROTEM-guided transfusion of fibrinogen concentrate during MOH can significantly reduce the need to transfuse these blood components.14-17 Therefore, using the ROTEM analyser to regularly monitor and accurately determine the coagulation state of MOH patients before transfusion could be instrumental in informing goal-directed transfusion therapy, with potential to improve clinical outcomes through early fibrinogen replacement therapy which reduces the transfusion of high volumes of other blood products.
Diagnosis of Coagulopathy at Point-of-Care Using the ROTEM Analyser
Rotational Thromboelastometry is a point-of-care coagulation testing method which measures the viscoelastic properties of clotting blood and provides a comprehensive picture from clot formation until the point of clot dissolution.21,22 It also provides information on platelet numbers, platelet function and fibrinogen reserves.23 Four assays; EXTEM, INTEM, FIBTEM and APTEM performed simultaneously assess different aspects of haemostasis to aid the diagnosis of coagulopathy. Abnormal clot amplitude and clot lysis profiles detected in the EXTEM and FIBTEM assays are consistently associated with a diagnosis of coagulopathy, high risk of bleeding and massive transfusion, and can also predict mortality.24 Hyperfibrinolysis identified by clot lysis index and maximum clot firmness in the EXTEM assay has been linked to increased mortality in trauma patients,24,25 while the FIBTEM assay successfully diagnosed hypofibrinogenemia of varying degrees and provided a guide for the transfusion of fibrinogen concentrate or its supplements.20,2628
Due to its rapid turnaround times and the ability to identify specific haemostatic defects/deficiencies to enable goal-oriented transfusion strategies, the ROTEM analyser is increasingly being used in diagnosis, monitoring and treatment of bleeding in high-risk patients such as those undergoing cardiac surgery, obstetric haemorrhage and during trauma-induced coagulopathy.4,15,21,24,26,28-35 Substantial data highlighting the advantages of point-of-care ROTEM assays have facilitated its incorporation into various treatment and diagnosis algorithms,15,18,31,35-38 leading to prompt diagnosis of coagulopathy during severe haemorrhage; reduced haemorrhage in patients requiring massive transfusion; and reduced numbers of transfusions, as well as the units/amounts of transfusion products used in surgery.26,28,30,34,35 The reduced blood product usage is largely attributed to early detection and treatment of coagulopathy, which in turn limits further blood loss that may arise from excessive or continued bleeding as standard laboratory test results are awaited.
In contrast, standard coagulation tests (PT, APTT, platelet count and Clauss fibrinogen) are inadequate in guiding transfusion therapy in emergencies due the longer turnaround times and the fact that they do not assess the comprehensive process of clot formation and dissolution.39 These tests may be of limited use particularly in patients with major obsteric haemorrhage who would require urgent assessment due to the increased risk of losing large volumes of blood relatively quickly, and the likelihood that their haemostatics may change rapidly with the onset of severe bleeding. Moreover, it has been suggested that that APTT and PT can remain normal during large bleeds of ≥1500 ml blood loss,6 suggesting that they are less likely to give an accurate early feedback about key changes in maternal haemostatic profile during MOH.
Methods
ROTEM-guided usage of blood products for the management of MOH was evaluated at the Royal Gwent Hospital, in South Wales. Ethical approval was granted by the Aneurin Bevan University Health Board and Cardiff Metropolitan University Ethics Committees. Usage of blood products based on decisions influenced by the ROTEM analyser tests was compared to blood product usage based on standard laboratory coagulation tests.
ROTEM-based cases (ROTEM group) comprised of 108 women with MOH attending the main delivery unit (MDU) from January 2017 to May 2018. Non-ROTEM based cases (Pre-ROTEM group) comprised 516 women with MOH who attended the MDU from January 2012 to December 2013. Pregnant women were included in the study if they experienced MOH (defined in this study as a blood loss ≥1000 ml) irrespective of whether or not they required transfusion of blood products. Obstetric women on anticoagulant therapy or those who did not experience a major haemorrhage were excluded. Blood product transfusion details were obtained from archived blood bank records.
The numbers of CRC, FFP, fibrinogen concentrate and platelets transfused was assessed by comparing the average rate of transfusion of each blood product (total number of products transfused, divided by the total number of women who were transfused) in the two groups. ROTEM measurements were validated by establishing the presence of a strong positive correlation between EXTEM-A5 and platelet count and a strong positive correlation of FIBTEM-A5 with fibrinogen concentration. Statistical analysis was performed by the GraphPad Prism statistical software. A p value <0.05 was considered statistically significant.
Results and Discussion
Validation of the ROTEM Analyser Data
The ROTEM’s FIBTEM assay correlates well with standard laboratory measures of fibrinogen concentration33 and is used to guide fibrinogen replacement therapy. Similarly, the EXTEM assay which measures the contribution of platelets to coagulation correlates with platelet counts and can predict thrombocytopenia and guide transfusion of platelet concentrates.40 Based on these observations, the clinical relevance of the ROTEM tests was ascertained in this study by testing for correlations of fibrinogen concentration with FIBTEM-A5, and platelet counts with EXTEM-A5 before analysing data for any possible impact on blood product usage. Consistent with published studies, FIBTEM-A5 and EXTEM-A5 correlated significantly with fibrinogen concentration (p<0.0001, Figure 1A) and platelet count (p < 0.0001 Figure 1B), respectively. The strong associations confirmed the expected performance of the ROTEM analyser, and validated its use in the current study.
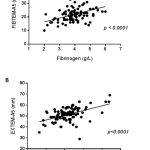 |
Figure 1: Correlation of ROTEM assays with Fibrinogen and platelet count.
|
The ROTEM analyser was used to measure coagulation parameters in 108 obstetric women with MOH in the ROTEM group. The values of FIBTEM-A5 and fibrinogen concentration (Panel A) and EXTEM-A5 and platelet counts (Panel B) were analysed for correlations using the linear regression method. A p value < 0.05 was considered statistically significant.
Blood Product Usage Based on ROTEM Analyser Measurements
In the ROTEM group, where the ROTEM analyser was used to manage MOH, 33.3% (36 of the 108 women) underwent transfusion therapy (Table 1). Of the 36 women receiving transfusion products, 28 women (78%) were transfused with a total of 60 units of CRC, making it the major blood component to be used (Table 1). This was followed by platelet transfusion at 22.2% (8 of 36 women transfused). Fibrinogen concentrate and FFP were the least transfused products in the ROTEM group. Of note, only 2 of the 108 women (1.9%) received fibrinogen concentrate, contrary to a published study which reported fibrinogen concentrate to be the most frequently administered haemostatic therapy for obstetric haemorrhage.41
Table 1: Average blood product usage per patient transfused in the ROTEM group.
| Parameter | ROTEM ANALYSER GROUP | |||
| Total number of women with MOH | 108 | |||
| Number women with MOH who received transfusion therapy | 36 (33.3%) | |||
| Average number of units of transfusion product per patient transfused ** | Blood Product | # of units transfused | # of patients transfused | Transfusion
rate |
| CRC | 60 | 28 (78%) | 2.14 | |
| FFP | 1 | 1 (2.8%) | 1 | |
| Fibrinogen Concentrate | 2 | 2 (5.6%) | 1 | |
| Platelets | 8 | 8 (22.2%) | 1 | |
| Total | 71 | |||
Notes/Key
CRC = Concentrated Red Cells; FFP = Fresh Frozen Plasma.
** Average units per patient transfused was calculated by dividing the total number of products transfused by the total number of women who were transfused.
The low rate (1.9%) of fibrinogen replacement therapy despite a significant level of obstetric haemorrhage (> 1000 ml blood loss) in the current study might be indicative of discrepancies in the measurement of blood loss and definition of MOH.5,15 Blood loss data from 43 women in the ROTEM group indicated varying degrees of blood loss: range = 1086 – 4600 ml; median = 1700; mean = 1850 ml (Table 2 & Figure 2), all consistent with MOH. However, the lower level of fibrinogen concentrate transfusions in this study is strongly supported by the absence of hypofibrinogenemia in obstetric women in the ROTEM group (Table 2 & Figure 2). Fibrinogen concentrate therapy is recommended only when FIBTEM-A5 levels fall to ≤ 11 mm and a hypofibrinogenemic state of fibrinogen levels <1.0 or 1.5 g/l is reached, 20,26,32,42,43 yet a majority of women in the current study had normal fibrinogen levels (reference range, 2-4 g/l), which did not meet the requirements for fibrinogen transfusion therapy. The average and median fibrinogen concentration in this study were 4 g/l (Table 2 & Figure 2) which are still within the typical range of 4-6 g/l expected for obstetric women in the 3rd trimester.44 Moreover, it has been shown that a fibrinogen concentration of at least 2.5 g/l can sufficiently maintain optimal haemostasis in post-partum haemorrhage.45,46 Furthermore, only two women in this study reached the threshold FIBTEM-A5 level of ≤11 (Figure 2), requiring fibrinogen therapy. This argument is further supported by the OBS2 study, a double-blind randomized controlled trial which showed that fibrinogen replacement was unnecessary in obstetric haemorrhaging women with a FIBTEM-A5 >12 mm or with fibrinogen levels > 2 g/l.47
Table 2. Summary of haemostatic measurements for women in ROTEM group.
| Parameter | Number of values | Minimum | Median | Maximum | Mean |
| Blood loss (ml) | 43 | 1086 | 1700 | 4600 | 1850 |
| APTT (s) | 93 | 20.2 | 25.6 | 36.9 | 25.8 |
| PT (s) | 93 | 9.3 | 10.4 | 12.2 | 10.5 |
| Fibrinogen (g/L) | 93 | 2 | 4 | 6 | 4 |
| Haemoglobin (g/L) | 43 | 63 | 102 | 147 | 104 |
| Platelets (x109/L) | 97 | 64 | 212 | 442 | 217 |
| FIBTEM-A5 (mm) | 108 | 10 | 22 | 45 | 22 |
| EXTEM-CT (s) | 108 | 34 | 51 | 79 | 51 |
| EXTEM-A5 (mm) | 108 | 29 | 52 | 69 | 52 |
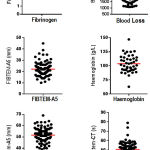 |
Figure 2: Distribution of various parameters in the ROTEM group.
|
Scatter dot plots showing the distribution of some of the parameters measured in women in the ROTEM group. Each dot represents an individual patient measurement. The horizontal line indicates the mean.
Blood Product Usage Based on Standard Laboratory Coagulation Tests
In the Pre-ROTEM group, transfusion therapy was initiated based on visual inspection of blood loss and haemostatic assessment by standard laboratory coagulation tests. Compared to the ROTEM group, 50.97% (263 of the 516 women) underwent transfusion therapy (Table 3). As with the ROTEM group, CRC was the major blood component transfused (95% of all transfused women received CRC), with a total of 711 units transfused to 250 women. However, unlike the ROTEM group, a considerably higher number of women (48/263, 18%) were transfused with FFP (a total of 203 units transfused to 28 women) compared to only 6.5% (17 of 263 women) who received platelets, with a total of only 25 units of platelet concentrates transfused. None of the women in this group received fibrinogen concentrate transfusion therapy.
Table 3: Average blood product usage per patient transfused in Pre-ROTEM group.
| Parameter | PRE-ROTEM GROUP | |||
| Total number of women with MOH | 516 | |||
| Number women with MOH who received transfusion therapy | 263 (50.97%) | |||
| Average number of units of transfusion product per patient transfused ** | Blood Product | # of units transfused | # of patients transfused | Transfusion
rate |
| CRC | 711 | 250 (95%) | 2.84 | |
| FFP | 203 | 48 (18%) | 4.23 | |
| Fibrinogen Concentrate | 0 | 0 (0%) | 0.00 | |
| Platelets | 25 | 17 (6.5%) | 1.47 | |
| Total | 939 | |||
Notes/Key
CRC = Concentrated Red Cells; FFP = Fresh Frozen Plasma
**Average units per patient transfused was calculated by dividing the total number of products transfused by the total number of women who were transfused.
Influence of ROTEM Haemostasis Analyser on Blood Product Usage
A comparison of the usage of various blood products between the ROTEM and Pre-ROTEM group indicated that a higher number of CRC, FFP and platelets were transfused per patient in the Pre-ROTEM group, and that using the ROTEM analyser led to a reduction in the transfusion of these products (Figure 3). This is consistent with published studies and shows that early detection and management of severe haemorrhage at point-of-care can effectively reduce the rate of blood loss and lead to a reduction in the numbers of patients transfused and volumes of CRC, FFP and platelets used.48,49 Reduction in platelet transfusions reflects the ROTEM analyser’s prompt and accurate detection of thrombocytopenia which can occur rapidly with ongoing MOH.
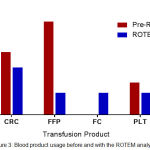 |
Figure 3: Blood product usage before and with the ROTEM analyser.
|
Usage of blood products before and after the introduction of point of care ROTEM analyser was compared by calculating the average units of blood products administered per person transfused. Average units per patient transfused was calculated by dividing the total number of products transfused by the total number of women who were transfused with a specific product for each group. Total number of women receiving any of the transfusion products is 263 for the Pre-ROTEM group and 36 for the ROTEM group. CRC; Concentrated Red Cells. FFP; Fresh Frozen Plasma. FC; Fibrinogen concentrate. PLT; Platelets.
The greatest reduction was seen in the transfusion of FFP (greater than 4-fold reduction), which may be largely a result of the early detection and management of blood loss based on the ROTEM tests such as the FIBTEM assay, which predicts blood loss based on accurate assessment of fibrinogen levels. However, it is important to note that correction of hypofibrinogenemia is increasingly using fibrinogen concentrate therapy (or cryoprecipitate) due to the low concentrations of fibrinogen in FFP (hence suboptimal haemostatic efficacy) and the inherent safety concerns of pooling FFP donations.50,51 This could be an additional factor to explain the higher usage of FFP in the Pre-ROTEM group, and the huge reduction seen in the ROTEM group. Although the data also suggested an increased transfusion of fibrinogen concentrate to women in the ROTEM group, the general absence of hypofibrinogenemia resulted in only two women receiving fibrinogen concentrate therapy. This small number of transfusions precludes any clinically relevant comparisons of fibrinogen usage with the Pre-ROTEM group.
Overall, there was 1.8-fold reduction in the total number of transfusion products administered to MOH women when transfusion was managed based on the ROTEM analyser measurements. This was calculated by dividing the total number of products transfused, by the number of patients transfused for each group (i.e. Pre-ROTEM group: 939/263 = 3.6; ROTEM group: 71/36 = 2.0), and calculating the ratio of Pre-ROTEM to the ROTEM group (3.6/2.0 = 1.8-fold reduction). It is possible that the ROTEM analyser enabled prompt and accurate assessment of haemostasis on the labour ward for women undergoing MOH, and facilitated early detection and correction of coagulopathy, which prevented the unnecessary transfusion of various blood products, including fibrinogen concentrate.
Conclusions and Future Perspectives
This study found that using the ROTEM analyser reduced the numbers of CRC, FFP and platelet units transfused to obstetric women experiencing MOH. A lower number of transfusions reflects the prompt and accurate assessment of the maternal coagulation state and the risk of bleeding, which prevented unnecessary use of blood products. Furthermore, accurate determination of fibrinogen levels by the ROTEM analyser led to relatively fewer fibrinogen concentrate transfusions in this study compared to published literature. This suggests a possibility for the ROTEM analyser to reduce blood product usage during MOH without necessarily increasing fibrinogen replacement therapy. These findings confirm that incorporation of specific ROTEM tests such as FIBTEM and EXTEM in MOH protocols has potential to improve the management of MOH and reduce the overall consumption of blood products, including fibrinogen concentrate. The findings encourage adoption of ROTEM-based coagulation testing in the routine monitoring and management of MOH, and call for further studies to assess the clinical efficacy of MOH protocols which incorporate ROTEM assays, with particular emphasis on quantitation of real-time fibrinogen levels in order to limit unnecessary fibrinogen replacement therapy.
Acknowledgements
I acknowledge the anaesthetists on the main delivery unit for enabling collation of data on the ROTEM tests and blood loss volume; and the transfusion laboratory team for their assistance in accessing archived transfusion records. This study was conducted as part of service evaluation and did not require informed consent. None of the participants have been identified in this publication.
References
- Brenner B. Ha emostatic changes in pregnancy. Thromb Res. 2004;114(5-6):409-414.
CrossRef - Cerneca F., Ricci G., Simeone R., Malisano M., Alberico S., Guaschino S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997;73(1):31-36.
CrossRef - Holmes V. A., Wallace J. M. Ha emostasis in normal pregnancy a balancing act? Biochem Soc Trans. 2005;33(2):428-432.
CrossRef - Carroll R. C., Craft R. M., Langdon R. J., Clanton C. R., Snider C. C., Wellons D. D., Dakin P. A., Lawson C. M., Enderson B. L., Kurek S. J. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154(1):34-39.
CrossRef - Charbit B., Mandelbrot L., Samain E., Baron G., Haddaoui B., Keita H., Sibony O., Mahieu-Caputo D., Hurtaud-Roux M. F., Huisse M. G., et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5(2):266-273.
CrossRef - de Lloyd L., Bovington R., Kaye A., Collis R. E., Rayment R., Sanders J., Rees A., Collins P. W. Standard haemostatic tests following major obstetric haemorrhage. Int J Obstet Anesth. 2011;20(2):135-141.
CrossRef - Chapin J. C., Hajjar K. A. Fibrinolysis and the control of blood coagulation. Blood reviews. 2015;29(1):17-24.
CrossRef - Chan A. K., Paredes N. The coagulation system in humans. Methods in molecular biology (Clifton.NJ). 2013;992:3-12.
CrossRef - Cortet M., Deneux-Tharaux C., Dupont C., Colin C., Rudigoz R. C., Bouvier-Colle M. H., Huissoud C. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108(6):984-989.
CrossRef - Collins P. W., Lilley G., Bruynseels D., Laurent D. B., Cannings-John R., Precious E., Hamlyn V., Sanders J., Alikhan R., Rayment R., et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage a prospective study. Blood. 2014;124(11):1727-1736.
CrossRef - Matsunaga S., Seki H., Ono Y., Matsumura H., Murayama Y., Takai Y., Saito M., Takeda S., Maeda H. A retrospective analysis of transfusion management for obstetric hemorrhage in a Japanese obstetric center. ISRN Obstet Gynecol. 2012;2012:854064.
CrossRef - Era S., Matsunaga S., Matsumura H., Murayama Y., Takai Y., Seki H. Usefulness of shock indicators for determining the need for blood transfusion after massive obstetric hemorrhage. J Obstet Gynaecol Res. 2015;41(1):39-43.
CrossRef - Bell S. F., Rayment R., Collins P. W., Collis R. E. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anesth. 2010;19(2):218-223.
CrossRef - Ahmed S., Harrity C., Johnson S., Varadkar S., McMorrow S., Fanning R., Flynn C. M. J.M., Byrne O. R. B. M. The efficacy of fibrinogen concentrate compared with cryoprecipitate in major obstetric haemorrhage–an observational study. Transfus Med. 2012;22(5):344-349.
CrossRef - Mallaiah S., Barclay P., Harrod I., Chevannes C., Bhalla A. Introduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in major obstetric haemorrhage. Anaesthesia. 2015;70(2):166-175.
CrossRef - Fenger-Eriksen C., Lindberg-Larsen M., Christensen A. Q., Ingerslev J., Sorensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101(6):769-773.
CrossRef - Matsunaga S., Takai Y., Nakamura E., Era S., Ono Y., Yamamoto K., Maeda H., Seki H. The Clinical Efficacy of Fibrinogen Concentrate in Massive Obstetric Haemorrhage with Hypofibrinogena emia. Scientific reports. 2017;7:46749.
CrossRef - Seto S., Itakura A., Okagaki R., Suzuki M., Ishihara O. An algorithm for the management of coagulopathy from postpartum hemorrhage, using fibrinogen concentrate as first-line therapy. Int J Obstet Anesth. 2017;32:11-16.
CrossRef - Fowler A., Perry D. J. Laboratory monitoring of haemostasis. Anaesthesia 2015;70(1):68-72, e24.
- Huissoud C., Carrabin N., Audibert F., Levrat A., Massignon D., Berland M., Rudigoz R. C. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG . an international journal of obstetrics and gynaecology. 2009;116(8):1097-1102.
CrossRef - Johansson P. I., Stissing T., Bochsen L., Ostrowski S. R. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45.
CrossRef - Whiting D., DiNardo J. A. TEG and ROTEM technology and clinical applications. American journal of hematology 2014;89(2):228-232.
CrossRef - Luddington R. J. Thrombelasto graphy thromboelastometry. Clin Lab Haematol. 2005;27(2):81-90.
CrossRef - Veigas P. V., Callum J., Rizoli S., Nascimento B., da Luz L. T. A systematic review on the rotational thrombelastometry (ROTEM(R)) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24(1):114.
CrossRef - Schochl H., Frietsch T., Pavelka M., Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma 2009;67(1):125-131.
CrossRef - Rugeri L., Levrat A., David J. S., Delecroix E., Floccard B., Gros A., Allaouchiche B., Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5(2):289-295.
CrossRef - Rourke C., Curry N., Khan S., Taylor R., Raza I., Davenport R., Stanworth S., Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342-1351.
CrossRef - Afshari A., Wikkelso A., Brok J., Moller A. M., Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;(3):7871.
- Armstrong S., Fernando R., Ashpole K., Simons R., Columb M. Assessment of coagulation in the obstetric population using ROTEM(R) thromboelastometry. Int J Obstet Anesth. 2011;20(4):293-298.
CrossRef - Coakley M., Reddy K., Mackie I., Mallett S. Transfusion triggers in orthotopic liver transplantation a comparison of the thromboelastometry analyzer the thromboelas togram and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20(4):548-553.
CrossRef - Lier H., Vorweg M., Hanke A., Gorlinger K. Throm boelastometry guided therapy of severe bleeding. Essener Runde algorithm. Hamostaseologie 2013;33(1):51-61.
CrossRef - Rahe-Meyer N., Solomon C., Winterhalter M., Piepenbrock S., Tanaka K., Haverich A., Pichlmaier M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. The Journal of thoracic and cardiovascular surgery. 2009;138(3):694-702.
CrossRef - Schochl H., Cotton B., Inaba K., Nienaber U., Fischer H., Voelckel W., Solomon C. FIB TE M provides early prediction of massive transfusion in trauma. Crit Care. 2011;15(6):265.
CrossRef - Shore-Lesserson L., Manspeizer H. E., DePerio M., Francis S., Vela-Cantos F., Ergin M. A. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88(2):312-319.
- Girdauskas E., Kempfert J., Kuntze T., Borger M. A., Enders J., Fassl J., Falk V., Mohr F. W. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. The Journal of thoracic and cardiovascular surgery 2010;140(5):1117-1124. e1112.
- Schochl H., Maegele M., Solomon C.l, Gorlinger K,. Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15.
CrossRef - Johansson P. I. Coagulation monitoring of the bleeding traumatized patient. Curr Opin Anaesthesiol 2012;25(2):235-241.
CrossRef - Collis R. E., Collins P. W. Ha emo static management of obstetric haemorrhage. Anaesthesia. 2015;70(1):78-86. e27-78.
- Dzik W. H. Predicting hemorrhage using preoperative coagulation screening assays. Curr Hematol Rep. 2004;3(5):324-330.
- Engberink O. R. H., Kuiper G. J., Wetzels R. J., Nelemans P. J., Lance M. D., Beckers E .A., Henskens Y. M. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(2):210-216.
CrossRef - Guasch E., Gilsanz F. Treatment of Postpartum Hemorrhage With Blood Products in a Tertiary Hospital: Outcomes and Predictive Factors Associated With Severe Hemorrhage. Clinical and applied thrombosis hemostasis official journal of the International Academy of Clinical and Applied Thrombosis Hemostasis. 2016;22(7):685-692.
CrossRef - Gorlinger K., Fries D., Dirkmann D., Weber C. F., Hanke A. A., Schochl H. Reduction of Fresh Frozen Plasma Requirements by Perioperative Point-of-Care Coagulation Management with Early Calculated Goal-Directed Therapy. Transfus Med Hemother. 2012;39(2):104-113.
CrossRef - Kozek-Langenecker S. A., Afshari A., Albaladejo P., Santullano C. A., Robertis D . E., Filipescu D. C., Fries D., Gorlinger K., Haas T., Imberger G., et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270-382.
CrossRef - Szecsi P. B., Jorgensen M., Klajnbard A., Andersen M. R., Colov N. P., Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103(4):718-727.
CrossRef - Levy J. H., Welsby I., Goodnough L. T. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion. 2014;54(5):1389-1405; quiz 1388.
CrossRef - Bolliger D., Szlam F., Molinaro R. J., Rahe-Meyer N., Levy J. H., Tanaka K. A. Finding the optimal concentration range for fibrinogen replacement after severe haemodilution: an in vitro model. Br J Anaesth. 2009;102(6):793-799.
CrossRef - Collins P. W., Cannings-John R., Bruynseels D., Mallaiah S., Dick J., Elton C., Weeks A. D., Sanders J., Aawar N., Townson J., et al. Viscoelastometric-guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double-blind randomized controlled trial. Br J Anaesth. 2017;119(3):411-421.
CrossRef - Rahe-Meyer N., Solomon C., Hanke A., Schmidt D. S., Knoerzer D., Hochleitner G., Sorensen B., Hagl C., Pichlmaier M. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery a randomized, placebo-controlled trial. Anesthesiology. 2013;118(1):40-50.
CrossRef - Rahe-Meyer N., Hanke A., Schmidt D. S., Hagl C. Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. The Journal of thoracic and cardiovascular surgery. 2013;145(3):S178-185.
CrossRef - Kozek-Langenecker S., Sorensen B., Hess J. R., Spahn D. R. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care 2011;15(5):239.
CrossRef - Yang L., Stanworth S., Hopewell S., Doree C., Murphy M. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. 2012;52(8):1673-1686; quiz 1673.









