Moushira Zaki1, Walaa Yousef 1, Sanaa Kamal1, Ramy Mohamed1, Omnia Saleh2 and Wafaa Ezzat2
1Biological Anthropology Department, Medical Research Division, National Research Centre, Giza, Egypt.
2Medicine Department, Medical Research Division, National Research Centre, Cairo, Egypt.
DOI : https://dx.doi.org/10.13005/bpj/1477
Abstract
Non-alcoholic fatty liver disease (NAFLD) is frequently related to obesity and metabolic alterations. This study aimed to assess the relation between NAFLD and metabolic syndrome (MS) in obese premenopausal women and investigate the impact of NAFLD on occurrence of metabolic syndrome components. The study comprised 180 non-smoking premenopausal obese women. They were 90 with NAFLD and 90 with normal liver, aged 25 to 35 years. Abdominal ultrasonography was used to diagnose fatty liver disease. MS was diagnosed according to the Adult Treatment Panel III criteria. Metabolic syndrome was found in 22.2% and in 83.3% of the normal and fatty liver cases, respectively, with significant difference. Cases with NAFLD had significantly higher levels of triglyceride, glucose, ALT, cholesterol, HOMA-IR and waist circumference than those than those with the normal fatty liver. In fatty liver group, the majority of cases had central obesity (88.8%), followed by hypertriglyceridemia (85.5%), hyperinsulinemia (84.4 %), hyperglycemia (83.3%) and hypertension (81.1%). All metabolic syndrome components were significantly elevated in the cases with fatty liver than those with in normal liver cases. The strongest associations of an individual component of metabolic syndrome with NAFLD were hypertriglyceridemia and low HDL-cholesterol. A higher percentage of NAFLD was observed in cases with three components followed by four components of the metabolic syndrome. NAFLD is correlated positively with metabolic risk components. It was associated with higher ratios of metabolic components; hypertriglyceridemia and low HDL-cholesterol level had the strongest positive association. This suggests the importance of these components in screening of NAFLD among obese premenopausal women.
Keywords
NAFLD; Metabolic Components; Obesity; Women
Download this article as:| Copy the following to cite this article: Zaki M, Yousef W, Kamal S, Mohamed R, Saleh O, Ezzat W. Association Between Metabolic Abnormalities and Non-Alcoholic Fatty Liver in Obese Premenopausal Women. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Zaki M, Yousef W, Kamal S, Mohamed R, Saleh O, Ezzat W. Association Between Metabolic Abnormalities and Non-Alcoholic Fatty Liver in Obese Premenopausal Women. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=21189 |
Introduction
NAFLD is the leading cause of chronic liver disease, with increasing in prevalence up to 20–30% worldwide.1 It is considered to be a hepatic manifestation of metabolic syndrome (MS) in which the central pathogenesis of NAFLD is insulin resistance.2 It has been reported to be associated with several features of metabolic syndrome including obesity, type 2 diabetes, atherogenic dyslipidemia, and hypertension, and is characterized by insulin resistance. Furthermore, NAFLD may be a hepatic manifestation of metabolic syndrome.3,4
The significant cause of morbidity in developed countries is NAFLD.5 In the general population its prevalence ranges from 10% to 24% and 75% in obese patients.6 The major causes of chronic liver damage is NAFLD which related to obesity, type 2 diabetes mellitus and other components of the metabolic syndrome.7 Across populations, the prevalence of MS varies widely. According to the National Health and Examination Survey (NHANES) III 1988-1994 and the NHANES 1999-2000, the age-adjusted prevalence rates of metabolic syndrome were 24.1% and 27%, respectively.8
Clinical and biochemical parameters revealed that insulin resistance play significant role in the pathogenesis of NAFLD. However, the impact of obesity and NALFD on the occurrence of metabolic syndrome has not been fully explained.
This study aimed to investigate the association between NAFLD and metabolic components in obese premenopausal women.
Methods
Study population
The study included 180 non-smoking women, aged 25 to 35 years (28.9 ± 2.3), 90 with NAFLD and 90 with normal liver , normal fasting glucose and normal liver function tests, all from Cairo. Diagnosis of NAFLD was based on ultrasound findings. All relevant investigations were done including lipid profile and liver function test.
This research has been approved by the Ethical Committee of National Research Centre, Egypt (number = 16361), in accordance with the World Medical Association’s Declaration of Helsinki.
Anthropometric Measurements
The weight of body and height were considered to the nearest 0.1 kg and 0.1 cm using an electronic scale and a stadiometer. Body mass index (BMI) was calculated as body weight/height2 (kg/m2). Waist circumference (WC) was determined at a point midway between the tenth rib and the iliac crest, recording in the centimeter [16]. Following at least ten minutes of rest blood pressure was measured twice, and the mean value was recorded.
Definition of the Metabolic Syndrome
MS was in women diagnosed when three or more of the five components were present, that is, (1) central obesity (waist circumference as defined by the Regional Office for the Western Pacific Region of the World Health Organization >80 cm ; (2) a triglyceride level ≥150 mg/dL; (3) < 50 mg/dL; (4) fasting glucose ≥ 100mg/dL or treatment for diabetes; (5) arterial pressure ≥ 130/85mmHg or treatment for hypertension.9
This study was conducted between December 2016 and March 2017 in the National Research Centre, Egypt; Medical Research Centre of Excellence. Age, fasting glucose, insulin, triglyceride (TG), and high-density lipoprotein (HDL) were recorded for the enrolled patients. Ultrasound was conducted for all patients and the findings of each case were recorded. The exclusion criteria included chronic hepatitis B and C. These patients were assigned to two groups according to whether they had a normal liver appearance (normal group) or a fatty liver (fatty liver group. Liver function was determined by assessing levels of serum alanine aminotransferase (ALT). The definition of upper normal limit (UNL) of ALT was 50 U/L in men and 35 U/L in women.
Insulin resistance was estimated using homeostasis model assessment (HOMA-IR).
The diagnosis of NAFLD was based on abdominal ultrasound examinations without including other causes of chronic liver disease (liver cirrhosis, hepatic carcinoma, hepatitis history, impaired hepatic function, hepatitis B, hepatitis and C virus infection or drugs for liver damage. Abdominal ultrasonographic examinations were performed by the same physician for all patients and controls using SonoAce R5 (6 MHz; Samsung).
Liver function was determined by assessing levels of serum alanine aminotransferase (ALT). The definition of upper normal limit (UNL) of ALT was 35 U/L in women.
Statistical Analysis
Mean and the standard deviation of for each of the measured parameters were expressed. MS and its components are expressed as a proportion in NAFLD patients as well as in the normal liver group. Chi-square test was used to assess the effects of ratio of metabolic syndrome components. Anthropometric measurements and individual serum values were analyzed by independent Student’s t test. Statistical significant difference was considered when P value below 0.05. Participants were divided into five groups according to the presence of total components of metabolic syndrome. To examine the strength of association between the risk factors and NAFLD, multivariate Cox’s regression was used with odds ratio (OR) and 95% confidence interval.
Results
Out of the 90 obese patients with NAFLD 75 (83.3%) had MS and 20 (22.2%) in normal liver group, showing a significant association between MS and NAFLD (p = 0.01) (Table 1).
Table 1: The relation between the presence of NAFLD and MS
| Presence of MS | Absence of MS | P | |
| NAFLD | 75 (83.3%) | 15 (16.6%) | .02 |
| Normal liver | 20 (22.2%) | 70 (77.7%) |
Table 2 shows a comparative analysis of clinical and biochemical characteristics between the NAFLD and with normal liver groups. The mean HDL-C was significantly lower in patients with NAFLD. Regarding the anthropometric measurements, WC was significantly higher in subjects diagnosed with NAFLD than normal liver cases. Furthermore, patients with NAFLD had a significantly higher means of HOMA-IR, FBG, TC, TG, LDL-C of AST and ALT levels.
| Characteristics | NAFLD
Mean ± SD |
Normal liver
Mean ± SD |
p |
| Age (years) | 28.1 ± 2.1 | 27.9 ± 3.7 | 0.61 |
| BMI(kg/m2) | 30.1 ± 2.56 | 30.9 ± 3.23 | 0.41 |
| WC (cm) | 111 ± 8.9 | 90.4 ± 7.1 | 0.02 |
| SBP (mmHg) | 139 ± 9.5 | 119.3 ± 8.4 | 0.02 |
| DBP (mmHg) | 89 ± 8.2 | 80.1 ± 7.2 | 0.02 |
| HOMA-IR | 5.6 ± 2.1 | 3.5 ± 1.2 | 0.02 |
| FBG (mg/dL) | 119 ± 27.9 | 98.6 ± 19.8 | 0.02 |
| TC (mg/dL) | 221 ±19.8 | 211.1±16.8 | 0.02 |
| TG (mg/dL) | 172 ± 29.9 | 137.9 ± 20.9 | 0.01 |
| HDL-C (mg/dL) | 61 ± 19.5 | 53 ± 11.5 | 0.01 |
| LDL-C | 160 ± 29.9 | 120.9 ± 22.2 | 0.01 |
| AST (U/L) | 38.3 ± 16.7 | 20 .6 ± 6.9 | 0.01 |
| ALT (U/L) | 57.9 ± 26.7 | 20.6 ± 14.7 | 0.01 |
Table 4 shows odds ratios and 95% confidence interval of associated fatty liver with MS, the strength of association between metabolic syndrome and fatty liver were significantly elevated in the cases with fatty liver. The presence of the metabolic syndrome was significantly associated with the metabolic components. The strongest predictive metabolic features for NAFLD were hypertriglyceridemia (OR, 4.8; 95% CI, 3.7-5.6) followed low HDL-cholesterol (OR, 4.1; 95% CI, 2.6 -3.1) then central obesity (OR, 2.7; 95% CI, 1.8-2.9) then hypertension (OR, 1.3; 95% CI, 1.6-1.9).Table 3 shows the relationship between NAFLD and components of the metabolic syndrome components. The associations between individual components of metabolic syndrome and fatty liver are presented in Table 3. Results showed significant positive associations between all metabolic components and presence of NFLD. The majority of cases had central obesity (88.8%), followed by hypertriglyceridemia hyperglycemia (85.5%), hyperinsulinemia (84.4 %) then hypertriglyceridemia (83.3%) and hypertension (81.1%).
Table 3: NAFLD and components of the metabolic syndrome components in obese women
| Characteristics | Variable | Obese
NAFLD Normal liver Yes n(%) No n(%) |
P | |
| WC (cm) | ≥ 80 cm | 80 (88.8%) | 32(35.5%) | 0 .01 |
| < 80 cm | 10 (11.1%) | 58(64.4%) | ||
| Blood pressure (mmHg) | >130/85 or on treatment | 73(81.1%) | 59(65.5%) | 0 .01 |
| < 130/85 | 17(18.8%) | 31(34%) | ||
| HOMA-IR | > 4.5 | 76 (84.4%) | 18 (20.0%) | 0 .001 |
| < 4.5 | 24(26.6%) | 72 (80.0%) | ||
| Fasting glucose (mg/dL) | >150 | 75(83.3%) | 20 (22.2%) | 0 .001 |
| ≤150 | 15 (16.6%) | 70 (77.7%) | ||
| HDL-C (mg/dL) | < 50 | 71(78.8%) | 30 (33.3%) | 0 .001 |
| > 50 | 19 (21.1%) | 60(66.7%) | ||
| Triglycerides (mg/dl) | ≥ 100 | 77(85.5%) | 22(24.4%) | 0 .001 |
| <100 | 15 (16.6%) | 68 (75.6%) | ||
Table 4: Association of fatty liver with metabolic syndrome components
| OR | 95% CI | |
| WC (cm) ≥ 80 cm | 2.7 | 1.8 – 2.9 |
| TG ≥ 150 | 4.8 | 3.7-5.6 |
| HDL-C< 50 mg/dL | 4.1 | 2.6 – 3.1 |
| FBG>100 mg/dL | 1.75 | 1.08 – 2.41 |
| Blood pressure ≥ 130/85 mmHg or on treatment | 1.3 | 1.6 – 1.9 |
Cases were divided according to the presence of total components of metabolic syndrome into five groups. The prevalence rates of NAFLD for the five components were 5%, 22%, 28%, 13% and 7%, respectively. Higher prevalence of NAFLD was associated with the presence of two or three features of metabolic syndrome (Fig. 1).
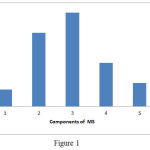 |
Figure 1
|
Discussion
NAFLD is defined as the hepatic component of metabolic syndrome.10–12 Therefore, increase of fat in hepatocytes is the key in the pathogenesis of NAFLD.13,14 Several studies showed that abnormal metabolic features is associated with NAFLD, including the increase in BMI, waist to hip ratio, diabetes and insulin resistance.11,12,15,16 Obesity, hypertriglyceridaemia and hypertension were found to be associated with NAFLD.17 Our study emphasized significantly higher prevalence of central obesity (88.8%), followed by hypertriglyceridemia (85.5%), hyperinsulinemia (84.4 %), hyperglycemia (83.3%) and hypertension (81.1%) in NAFLD cases than subjects normal liver. The incidence of NAFLD reached 60% −90% in obese cases, thought to be closely related to insulin resistance and in the hepatic symptoms of metabolic syndrome.4 The prevalence of NAFLD was found to be 43% in impaired fasting glucose cases and was 62% in the type 2 diabetes mellitus.18 Moreover, hypertriglyceridemia and low HDL-cholesterol level were found in 64% and 42% of NAFLD patients, respectively.19 The prevalence of NAFLD in individuals with dyslipidemia attending lipid clinics was estimated to be 50%.20
Previously, central obesity was found to be significantly associated with NAFLD in obese OR = 1.97, 95%CI: 1.38-2.80), and in non obese cases (OR = 2.17, 95%CI: 1.17-4.05). Moreover, diabetes, hypertension and metabolic syndrome are associated with NAFLD.21 Our study showed that the strongest predictive metabolic features for NAFLD were hypertriglyceridemia (OR, 4.8; 95% CI, 3.7-5.6) followed low HDL-cholesterol (OR, 4.1; 95% CI, 2.6 -3.1) then central obesity (OR, 2.7; 95% CI, 1.8-2.9) then hypertension (OR, 1.3; 95% CI, 1.6-1.9). In addition, previous study reported that metabolically obese NAFLD patients had high body fat percentage and lesser lean body mass and larger waist circumference.22 Also, higher sensitivity of NAFLD for insulin resistance than that for MS has reported.23 In our study the majority of cases had central obesity (88.8%), followed by hypertriglyceridemia (85.5%), hyperinsulinemia (84.4 %), hyperglycemia (83.3%) and hypertension (81.1%). Generally obesity is the most significant factor contributes in the development of metabolic syndrome. However, other studies emphasized that the fatty liver disease is risk factor for the development of insulin resistance and metabolic syndrome.24–26 The current study showed higher percentage of NAFLD in cases with three components followed by four components of the metabolic syndrome.
The liver is the site of the production of glucose and very-low-density lipoproteins that contain the majority of triglycerides. This involvement means that MS and NAFLD share the same risk profiles.27 Previous studies have indicated that dietary fat and adipocyte lipolysis increase hepatic fat in patients with insulin-resistant without invoking insulin-driven de novo hepatic lipogenesis.28,29 In this context, decreasing caloric intake will allow decrease postprandial lipid loading of the liver.
In conclusion, our findings showed different metabolic factors for NAFLD in premenopausal women with highest prevalence in women with two or three features of the metabolic syndrome. The study emphasized the importance of metabolic parameters in the pathogenesis of NAFLD in obese premenopausal women. Hypertriglyceridemia and low HDL-cholesterol are the strongest positive predive features for MS in NAFLD and NAFLD can be used as a predictor for metabolic syndrome and insulin resistance.
Acknowledgments
This study was supported by a grant from National Research Centre, Egypt.
References
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155-161.
CrossRef - Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844-1850.
CrossRef - Gianotti G, Cenni A, Bianchi G, et al. Diastolic dysfunction and cardiovascular risk in old subjects: possible association with NAFLD? Arch Gerontol Geriatr. 2014;58(2):188-195.
CrossRef - Dowman J.K, Tomlinson J.W, Newsome P.N. Systematic review: the diagnosis and staging of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33(5):525-540.
CrossRef - Koppe S.W.P, Sahai A, Malladi P, Whitington P.F, Green R.M. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41(4):592-598.
CrossRef - Clark J.M, Diehl A.M. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124(1):248-250.
CrossRef - Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology. 2006;130(6):1848-1852.
CrossRef - Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care. 2004;27(10):2444-2449.
CrossRef - Expert Panel on Detection E. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Jama. 2001;285(19):2486.
CrossRef - Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44-52.
CrossRef - Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112-117.
CrossRef - Byrne C.D, Olufadi R, Bruce K.D, Cagampang F.R, Ahmed M.H. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci. 2009;116(7):539-564.
CrossRef - Marceau P, Biron S, Hould F-S, et al. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84(5):1513-1517.
CrossRef - Chaves G.V, Souza D.S de, Pereira S.E, Saboya C.J, Peres W.A.F. Association between non-alcoholic fatty liver disease and liver function/injury markers with metabolic syndrome components in class III obese individuals. Rev Assoc Med Bras. 2012;58(3):288-293.
CrossRef - Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35(2):367-372.
CrossRef - Ahmed M.H, Abu E.O, Byrne C.D. Non-Alcoholic Fatty Liver Disease (NAFLD): new challenge for general practitioners and important burden for health authorities? Prim Care Diabetes. 2010;4(3):129-137.
CrossRef - Tsuneto A, Hida A, Sera N, et al. Fatty liver incidence and predictive variables. Hypertens Res. 2010;33(6):638.
CrossRef - Jimba S, Nakagami T, Takahashi M, et al. Prevalence of non‐alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22(9):1141-1145.
CrossRef - Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917-923.
CrossRef - Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45(10):1929-1934.
CrossRef - Feng R-N, Du S-S, Wang C, et al. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol WJG. 2014;20(47):17932.
CrossRef - Kwon Y-M, Oh S-W, Hwang S, Lee C, Kwon H, Chung G.E. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107(12):1852.
CrossRef - Sinn D.H, Gwak G-Y, Park H.N, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107(4):561.
CrossRef - Marchesini G, Brizi M, Morselli-Labate A.M, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450-455.
CrossRef - Younossi Z.M, Diehl A.M, Ong J.P. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology. 2002;35(4):746-752.
CrossRef - Bugianesi E, Moscatiello S, Ciaravella M.F, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16(17):1941-1951.
CrossRef - Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. lancet Diabetes Endocrinol. 2014;2(11):901-910.
CrossRef - Donnelly K.L, Smith C.I, Schwarzenberg S.J, Jessurun J, Boldt M.D, Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343-1351.
CrossRef - Vatner D.F, Majumdar S.K, Kumashiro N, et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci. 2015;112(4):1143-1148.
CrossRef








