Wassef Girgiss Nicola1, Mina Wassef Girgiss1, Aly Mohamed Ezz El-Arab2, Dawoud Fakhry Habib3, Mohamed Elsayed Elnemr4, Nadia Mohamed Ahmed3 and Eman Refaat Youness3
1Internal Medicine, Endocrinology and metabolism-Department of Internal Medicine, Medical Division, National Research Centre (NRC), Cairo, Egypt.
2Nutrition-Department of Nutrition and Food Science, National Research Centre (NRC), Cairo, Egypt.
3Biochemistry-Medical Biochemistry Department, Medical Division, National Research Centre (NRC), Cairo, Egypt.
4Internal Medicine, Department of Internal Medicine, 6th October University-Egypt.
Corresponding Author E-mail: mwgnicola@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1435
Abstract
Type 2 diabetic microangiopathy affects every organ in the body and can lead to serious incapacitating complications. VLDL and apo C1 are two of the main biochemical abnormalities which start and propagate this condition. Inulin fructans prebiotic effect on the colonic flora enhance the bifidogenic strains. These predominate over the pathogenic strains which encourage lipidogenesis, thus reducing hyperlipidemia. Our aim is to find out the possible effect of inulin ingestion on the metabolism of VLDL and apo C1 and their role in the pathogenesis of diabetic angiopathy Twenty eight obese type 2 diabetic female patients were subjected to this study. Each patient ingested 4 grams of inulin daily for 3 weeks. Their fasting serum level of VLDL and apo C1 were estimated before and after the period of inulin ingestion. There was a significant decrease in fasting level of serum VLDL and apo C 1 after inulin ingestion period. In conclusion inulin can be given as a protective and as an add on therapy for type 2 diabetic patients. It reduces two of the main culprits which start and propagate the pathologic pathway of diabetic microangiopathy. This cuts short the other offenders (small HDL, small dense LDL and the small VLDL remnants).
Keywords
Apo C1; Inulin; Microvasculopathy; Triglycerides; Type 2 Diabetes; VLDL
Download this article as:| Copy the following to cite this article: Nicola W. G, Girgiss M. W, El-Arab A. M. E, Habib D. F, Elnemr M. E, Ahmed N. M, Youness E. R. Protective Effect of Inulin and the Integrity of the Microvasculature in Diabetes Mellitus. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Nicola W. G, Girgiss M. W, El-Arab A. M. E, Habib D. F, Elnemr M. E, Ahmed N. M, Youness E. R. Protective Effect of Inulin and the Integrity of the Microvasculature in Diabetes Mellitus. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=21163 |
Introduction
Diabetic microvascular disease is usually associated with complications in the various body organs. On the long run this can lead to damage, deterioration and eventually end organ failure in the different body systems. Diabetic microvasculpathy has long been recognized to predominate in type 1 rather than in type 2 diabetics.1,2 However, several studies reported that diabetic arteriolosclerosis is as common in type 2 as in type 1 diabetic patients.3,4
Hyperglycemia which is a key feature of both type 1 and type 2 diabetes,5 is not the only determining factor behind the vascular complications. Trials for the tight control of hyperglycemia, reduced the progression of diabetic microvascular disease. Despite this fact, the morbidity and complications of diabetic arteriolosclerosis are still rising.1,2,6,7
Beside hyperglycemia, several factors participate in the pathogenesis and development of diabetic microvasculopathy. These include oxidative stress, systemic (metabolic) inflammation, protein synthesis in the extracellular matrix together with thickening of the capillary basement membrane.8
Moreover, very low density lipoprotein (VLDL) is also an important and outstanding determinant in the pathogenesis and progression of the microvascular disease in diabetes mellitus. VLDL is considered a major and independent participant in this regard.9,10 Also, it has been observed that agents which act through perioxisome proliferator activated receptor alpha (PPARα) to lower serum triglycerides (TG) which are carried mainly by VLDL have a beneficial therapeutic effect on diabetic nephropathy.11
Again, apolipoprotein C1 (apo C1) is an important component of the surface protein monolayer which envelops the lipid core of VLDL and high density lipoprotein (HDL) particles. These latter two lipoproteins are the main carriers of apo C1 which is secreted by the liver.12 In human plasma, apo C1 acts as a physiological regulator of the cholesteryl ester transfer protein (CETP) activity. It regulates the transfer of cholesteryl esters between the different lipoproteins, mainly VLDL, HDL and low density lipoprotein (LDL).12 In normolipidemic persons, apo C1 regulates the actions of HDLs with CETP.13,14 In hypertriglyceridemic subjects however, apo C1 acts in an angiopathogenic manner.15
Ingestion of the oligosaccharide inulin fructans has been known to decrease the synthesis of triglycerides.16,17 This oligosaccharide escapes digestion in the upper gastro-intestinal tract. In the colon, inulin is consumed by the bifidogenic bacteria, these latter supercedes the pathogenic strains which encourage lipogenesis.18,19
The aim of the present study is to find out the possible effect of inulin ingestion on the metabolism of VLDL and apo C1 and their role in the pathogenesis of diabetic angiopathy. Reviewing the literature and to the best of our knowledge; this study has not been tried on human subjects before.
Material and Methods
Subjects
Twenty eight obese, type 2 diabetic women were recruited from the municipal hospitals of Cairo, Egypt. The age range was 40-65 years.
Inclusion criteria: obese (body mass index > 30), type 2 diabetic females, middle aged or above, hypertensive or not and not under antilipidemic drugs.
Exclusion criteria:
Hormonal therapy or contraceptive pills. Endocrine disorders or other metabolic diseases.
Malignancy or organ failure (lung, heart, liver or Kidney). Local or systemic infection including chest, urinary tract, gastro-intestinal tract or skin infection (local or extensive).
Ethical Committee Approval
The present work has been approved by the ethical committee of the National Research Centre (NRC), Cairo, Egypt. Certificate number 15011.
Consent
All patients signed consent for participation.
Methods
Inulin fructans type prebiotic was given to the patients as an add on therapy to their conventional antidiabetic treatment. Four grams of inulin were given with milk to each patient daily; 2 grams in the morning and 2 grams in the evening for twenty one consecutive days. This dose was chosen empirically on the assumption that a small amount of inulin can exert a bifidogenic effect.20
Inulin specifications: Inulin A.R (C6H10O5) N ALPHA-CHEMIKA Mumbai. 400002 (INDIA) An ISO: 9001: 2000 Certified company.
All patients were subjected for the following before and after the period of inulin intake.
A-Full history. B- Thorough clinical examination. C- Laboratory investigations:
Laboratory Investigations
Estimation of fasting serum very low density lipoprotein (VLDL). This was done by dividing fasting serum triglycerides in mg/dl by 5 using Friedewald equation for calculating VLDL and LDL.21
Fasting serum triglycerides were estimated spectrophotometrically after Fossati (Fossati, 1982). Kit used from Centronic Germany.22
Estimation of fasting serum apolipoprotein C1 (apo C1) was by ELISA method after Westerterb et al 2007.23
Kit used: “Human Apolipoprotein C-1 ELISA Kit” was used from Assaypro LLC 3400 Harry’s Truman Blvd st. Chales, MO 63301.
Statistical Methods
Data were coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp. Chicago, USA, 2013.
Descriptive statistics were done for quantitative data as minimum and maximum of the range as well as mean± standard deviation (SD) for quantitative parametric data. Inferential analysis of quantitative variables using paired t-test in cases of two dependent groups with parametric data was performed. The level of significance was taken at P value < 0.05.
Results
Mean fasting serum level of very low density lipoprotein (VLDL) dropped significantly after inulin ingestion from 46.7+21.1 mg/dl to 39.6+18.8 mg/dl with P 0.005, table (1) and figure (1).
Mean fasting serum apolipoprotein C1 (apo C1) dropped significantly after inulin ingestion from 0.44+0.2 ug/ml to 0.29+0.09 ug/ml with P < 0.001 table (1) and figure (2).
Table 1: Shows the values of VLDL and apo C1 before and after inulin ingestion period.
| Before | After | ^Change | #P | ||||
| Mean±SD | Range | Mean±SD | Range | Median (IQR) | Range | ||
| VLDL (mg/dL) | 46.7±21.1 | 12.4–86.8 | 39.6±18.8 | 16.0–78.4 | -8.7 (-12.3–-3.3) | -25.6–43.6 | 0.005* |
| APOC I (µg/mL) | 0.44±0.20 | 0.14–0.72 | 0.29±0.09 | 0.10–0.49 | -0.15 (-0.19–-0.02) | -0.44–0.16 | <0.001* |
N=28, ^Negative values indicate reduction, #P-value of paired t-test, *Significant
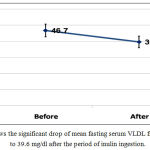 |
Figure 1: shows the significant drop of mean fasting serum VLDL from 46.7 mg/dl to 39.6 mg/dl after the period of inulin ingestion.
|
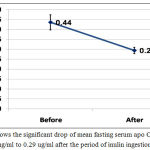 |
Figure 2: Shows the significant drop of mean fasting serum apo C1 from 0.44 ug/ml to 0.29 ug/ml after the period of inulin ingestion.
|
Discussion
In diabetes mellitus, pathophysiological disorders start years before manifest pathological changes appear in the microvessels and decades before hyperglycemia is detected.24 Unlike the macrovascular changes, atheromatous lesions are not characteristic for microvessels. The indicative changes of diabetes mellitus in microvasculopathy are extracellular matrix deposition, thickening of their walls and the basement membrane of the capillaries.8 These complications affect almost every organ in the body.25
Diabetes is a leading cause of blindness worldwide. Diabetic retinopathy (DR) is a well known microvascular diabetic complication; which when neglected it progresses to complete loss of vision.26 The retina is a part of the central nervous system (CNS). Both of them share anatomical and embryologic features. The presence of DR alerts for searching other vascular CNS complications.27 Also, affection of the vasanervorum is among the causes of diabetic neuropathy.5 Again, diagnosis of DR anticipates the presence or the near occurance of diabetic nephropathy.28,29 This latter starts by glomerular hyperfilteration which progresses to microalbuminurea, macroalbuminurea, renal impairment up to end stage renal failure.28,29 Diabetic microangiopathy, also leads to other dreadful complications; Diabetic cardiomyopathy can occur in the presence of patent coronary arteries.30,31 However, when the vasa-vasorum are affected, this aggravates the atherosclerosis of the large vessels; the coronaries or elsewhere.32,33 Diabetic microangiopathy leads to special entity of complications termed microamputations.34 In the skin microvasculopathy is behind delayed wound healing, gangrenous ulcerations and aggravated skin infections.5
In normolipidemic persons apo C1 acts as a normal physiologic regulator of lipid metabolism.13,14 In hypertriglyceridemic subjects, apo C1 acts in a pathological manner15. Hypertriglyceridemia and excess VLDL are features of type 2 diabetes mellitus. Elevated VLDL together with its integral moiety of apo C1 are key factors in the pathological sequences of type 2 diabetic vasculopathy. Apo C1 plays a critical metabolic role in this regard. It affects in an abnormal manner the metabolism of both VLDL, HDL and the redistribution of their triglycerides and cholesteryl ester content. Apo C1 stimulates VLDL hepatic synthesis.35 Also, it inhibits the lipoporotein lipase which hydrolyses VLDL.36,37 At the same time it inhibits the recognition of VLDL by their cellular receptors; It hinders the ligand (apo E) present on the surface of VLDL particles from binding to their receptors in the liver. This delays the catabolism of these particles and retards their clearance from the circulation.38,39 The net result of the above pathological steps is the accumulation of VLDL in the blood stream. These accumulating VLDL particles interact with HDL and LDL in a pathological manner15.
Concerning HDL, the accumulating apo C1 (integral to the abundant VLDL particles), readily dissociate from VLDL and rapidly associate with HDL particles. Being a strong inhibitor of CETP, apo C1 reduces the capacity of CETP in the HDL for transferring its cholesteryl esters (CE) to VLDL in exchange of the latter’s triglycerides.15,40 This results in “deranged triglycerides (TG)/ high density lipoprotein (HDL) axis”.41,42 The net result is the accumulation of this CE in the HDL turning it into an abnormally functioning particle. LDL particles are subjected to a similar mechanism like that to which HDL particles are exposed.41,42
The prolonged stay of VLDL particles in the circulation (as a result of the defective and delayed catabolism); delivers excess of their TG content to HDL and LDL. Upon reaching the liver, TG rich HDL & LDL particles are exposed to the hepatic lipoprotein lipase enzyme (LPL) which hydrolyses their TG content converting them to small HDL particles and small dense atherogenic LDL particles. The first are lost in urine while the latter easily percolate through the vascular endothelium, become readily oxidized and engulfed by the macrophages. This starts and perpetuates the damaging inflammatory process in the vessel wall.43
After the transfer of their TG to both LDL and HDL, the donor VLDL particles lose size. When the small size VLDL particles enter the different vascular beds, their TGs are hydrolyzed by the lipoprotein lipase enzyme in the (liver, skeletal muscles, myocardium and adipose tissue). The new small particles are termed VLDL remnants. These are proinflammatory as they behave like small LDL particles.
This study examined the effect of inulin intake on serum level of very low density lipoprotein (VLDL) and apolipoprotein C1 (apo C1). Inulin ingestion significantly reduced both VLDL and apo C1. These two compounds are heavily implicated in the pathogenesis of diabetic microangiopathy.9,10,15
Conclusions
In conclusion, inulin may help to reverse the pathogenic process of diabetic microangiopothy by lowering both VLDL and apo C1. Lower levels of these two compounds, in turn, decrease the proinflammatory small dense LDL particles and the proinflammatoty small VLDL remnants. Also, by improving the deranged TG/ HDL axis, inulin reduces the production of the ineffective small HDL particles which are lost in urine. Thus inulin which is a safe and edible fiber, can be given to diabetic subjects as both a protective and as an add on therapy for diabetic microangiopathy.
This study should be extended on a wider scale including a greater number of patients and a longer duration of inulin intake. At the same time end points should be incorporated in the study including number of sessions of laser photocoagulation in patients with diabetic retinopathy. Also serum creatinin, estimated glomerular filteration rate (eGFR) and urinary albumin in patients with diabetic nephropathy. All these should be determined before and after inulin intake period
Acknolwdgements
Thanks for National Research Center, Cairo, Egypt for financing this work with project number 10010312. Again, thanks to all patients who volunteered to participate in this study.
Conflict of Interest
There is no conflict of interest.
References
- Nathan D.M, Cleary P.A, Backlund J.Y, Genuth S.M, Lachin J.M, Orchard T.J, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653.
CrossRef - The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependant diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
CrossRef - Bash L.D, Selvin E, Steffes M, Coresh J, Astor B.C. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminurea and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008;168:2440.
CrossRef - Klein R, Klein B.E, Moss S.E, Cruickshanks K.J. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch Intern Med. 1994;154:2169.
CrossRef - Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J. Am Coll Cardiol. 2009;53(5 Suppl):35-42.
CrossRef - Advance Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J. Med. 2008;358:2560-2572.
CrossRef - Holman R.R, Paul S.K, Bethel M.A, Matthews D.R, Neil H.A: 10-year follow up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
CrossRef - Chawla A, Chawla R, Jaggi S. Microvascular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;Jul-Aug 20(4):456-551.
CrossRef - Muntner P, Coresh J, Smith J.C, Eckfeldt J, Klag M.J. Plasma lipids and risk of developing renal dysfunction: the Atherosclerosis Risk in Communities study. Kidney Int. 2000;58:293-301
CrossRef - Anami Y, Kobori S, Sakai M, Kasho M, Nishikawa T, Yano T, et al. Human beta-migrating very low density lipoprotein induces foam cell formation in human mesangial cells. Atherosclerosis. 1997;135:225-234.
CrossRef - Balakumar P, Kadian S, Mahadevan N. Are PPARα agonists a rational therapeutic strategy for preventing abnormalities of the diabetic kidney? Res. 2012;65:430-436.
- Cohn J.S, Tremblay M, Batal R, Jacques H, Veilleux L, Rodriguez C, et al. Plasma Kinetics of VLDL and HDL apo C-1 in normolipidemic and hypertriglyceridemic subjects. J Lipid Res. 2002;43:1680-1687.
CrossRef - Bjorkegren J, Boquist S, Samnegard A, Lundman P, Tornvall P, Ericsson CG, et al. Accumulation of apolipoprotein C-1-rich and cholesterol-rich VLDL remnants during exaggerated postprandial triglyceridemia in normolipidemic patients with coronary artery disease. 2000;101:227-230.
- Dautin G, Soltani Z, Ducloux D, Gautier T, Pais de Barros J.P, Gambert B, et al. Hemodialysis reduces plasma apolipoprotein C-1 concentration making VLDL a better substrate for lipoprotein lipase. Kidney Int. 2007;72:871-878.
CrossRef - Dumont L, Gautier T, Pais de Barros J.P, Laplanche H, Blache D, Ducoroy B, et al. Molecular mechanism of the blockade of plasma cholesteryl ester transfer protein by its physiological inhibitor apolipoprotein C 1. J Biol Chem. 2005;280:38108-38116.
CrossRef - Larsen N, Vogensen F.K, van den Berg F.W, Nielsen D.S, Andreasen A.S, Pederson B.K, et al. Gut microbiota in human adults with type 2 diabetes differ from non-diabetic adults. PLoS One. 2010;5:e9085.
CrossRef - Collado M.C, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894-899.
CrossRef - Santacruz A, Collado M.C, Garcia-Valdes L, Segura M.T, Martin-Lagos J.A, Anjos T, Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83-92.
CrossRef - Schwiertz A, Taras D, Schafer K, Beijer S, Bos N.A, Donus C, et al. Microbiota and SCFA in lean and overweight heathy subjects. Obes Silver Spring. 2010;18:190-195.
CrossRef - Kelly G. lnulin-type prebiotics — a review part 1. Altern Med Rev. 2008 Dec;4(13):315-329.
- Friedewald W.T, Levy R1, Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- Fossati P. Enzymatic determination of serum triglycerides. Principal Clin Chem. 1982;28:2077-2084.
- Westerterp M, Berbee J.F.P, Delsing D.J.M, Jong M.C, Gijbels M.J.J, Dahlmans V.E.H, et al. Apolipoprotein C-1 binds free fatty acids and reduces their intracellular esterification. J Lipid Research. 2007;48:1353-1361 .
CrossRef - Steffel J, Luscher T.F. Predicting the development of atherosclerosis. 2009;119:919-921.
- Standards of medical care in diabetes- 2016: Summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4-S5.
- Chawla A, Chawla R, Chawla A. Correlation Between Retinopathy and Other Modifiable Risk Factors. Presented on American Diabetes Association’s 75th Scientific Session. Boston; Massachusetts. 2015June;5-9.
- Wong T.Y, Klein R, Couper D.J, Cooper L.S, Shahar E, Hubbard L.D, et al. Retinol microvascular abnormalities and incident stroke: The atherosclerosis risk in communities study. 2001;358:1134-1140.
- Gross J.L, de Azevedo M.J, Silveiro S.P, Canani L.H, Caramori M.L, Zelmanovitz. Diabetic nephropathy: Diagnosis prevention and treatment. Diabetes care. 2005;28:164-176.
CrossRef - Adler A.I, Stevens R.J, Manley S.E, Bilous R.W, Cull C.A, Holman R.R. UKPDS Group. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int . 2003;63:225-232.
CrossRef - Unger R.h, Clark G.O, Scherer P.E, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochimica et Biophysica Acta. 2010;1801(3):209-214.
CrossRef - Bulinilkunnil T, Rodrigues B. Cardiac lipoprotein lipase: metabolic basis for diabetic heart disease. Cardiovascular Research. 2006;69(2):329-340.
CrossRef - Fowler M.J. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 26:77-82 Hagg S, Thorn L.M, Butaala J, Liebkind R, Harjutsalo B, Forsblom C.M. et al. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36:4140.
CrossRef - Rajamani K, Colman P.G, Li L.P, Best J.D, Voysey M.D’ Emden M.C, et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespectified analysis of a randomized controlled .Lancet. 2009;373:1780-1788.
CrossRef - Westerterb M, de Haan W, Berbee J.F, Havekes L.M, Rensen B. Endogenous apo C-1 increases hyperlipidemia in apo E-Knockout mice by stimulating VLDL production and inhibiting LPL. J Lipid Res. 2006;47:1203-1211.
CrossRef - Berbee J.F, van der Hoogt C.C, Sundararaman D, Havekes L.M, Rensen P.C. Severe hypertriglyceridemia in human APO C1 transgenic mice is caused by apo C-1-induced inhibition of LPL. J Lipid Res. 2005;46:297-306.
CrossRef - Cond-Knape K, Bensadoun A, Sobel J.H, Cohn J.S, Shachter N.S. Overexpression of apo C-1 in apo E-null mice: severe hypertriglyceridemia due to inhibition of hepatic lipase. J Lipid Res. 2002;43:2136-2145.
CrossRef - Swaney J.B, Weisgraber K.H. Effect of apolipoprotein C-1 peptides on the apolipoprotein E content and receptor binding properties of beta – migrating very low density lipoproteins. J Lipid Res. 1994;35:134-142.
- Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991;266:18259-18267.
- Gautier T, Masson D, pais de Barros J.P, Athias A, Gambert B, Aunis D, et al. Human apolipoprotein C-1 accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J Biol Chem. 2000;275:37504-37509.
CrossRef - Henry N.G, Yuan L.Z, Antonio H.O. Regulation of plasma triglycerides in insulin resistance and diabetes. Archives of Medical Research. 2000;36:232-240.
- Szapary P.O, Radar D.J. The triglyceride-high density lipoprotein axis: An important target of therapy? Am Heart J. 2004;148:211-221.
CrossRef - Leitinger N. Oxidised phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421-430.
CrossRef








