A. O. Zeltukhin1, G. V. Ilyinskaya1,2, A. V. Budanov1,3 and P. M. Chumakov1,4
1Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia.
2Blokhin Cancer Research Center, Moscow, Russia.
3Trinity Biomedical Sciences Institute, Trinity College, Dublin, Ireland.
4Chumakov Institute of Poliomyelitis and Viral Encephalitides, Federal Scientific Center for Research and Development of Immune-Biology Products, Russian Academy of Sciences, Moscow, Russia.
DOI : https://dx.doi.org/10.13005/bpj/1430
Abstract
In mammals a small family of genes called Sestrins play important roles in the maintenance of metabolic and redox homeostasis, suggesting that the genes may positively affect the lifespan and counteract the age-related functional decline. The nematode genome contains a single cSesn gene that makes the Caenorhabditis elegans an excellent model for studying functions of the sestrin family. We describe phenotypic differences of worms that have compromised expression of cSesn gene. By comparing three different cSesn-deficient modes with the wild-type C. elegans strain we show that the abrogation of cSesn expression results in an increased body size, an extended period of body growth, a reduces brood size and number of offspring per a single worm, an accelerated decline in muscular functions revealed as a rapid decrease in the pharyngeal pumping rate and in the overall locomotory activity. The results are consistent with the potential roles of cSesn in counteracting the process of aging in C. elegans.
Keywords
Aging; Age-Related Body Size; Caenorhabditis Elegans; Manifestations Metabolism;Sestrin Gene; Stress Resistance;
Download this article as:| Copy the following to cite this article: Zeltukhin A. O, Ilyinskaya G. V, Budanov A. V, Chumakov P. M. Some Phenotypic Characteristics of Nematode Caenorhabditis Elegans Strains with Defective Functions of the Sestrin (cSesn) Gene. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Zeltukhin A. O, Ilyinskaya G. V, Budanov A. V, Chumakov P. M. Some Phenotypic Characteristics of Nematode Caenorhabditis Elegans Strains with Defective Functions of the Sestrin (cSesn) Gene. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20423 |
Introduction
Nematode Caenorhabditis elegans is a tiny free-living worm that has a transparent body with strictly defined number of cells and simple requirements for laboratory cultivation. It has been chosen as a convenient model for studying genetics and physiology of multicellular organisms.1 During the decades of intensive studies C. elegans has became an invaluable model for studies in reproductive, developmental and metabolic regulation, neurobiology, molecular physiology, pharmacology, environmental biology and in many other areas. The availability of complete genome structure, the simplicity of manipulations with gene expression, a wide array of available mutants in the majority of genes and many genetic instruments provided by the scientific community make C. elegans one of the most useful and popular animal models.2 The relatively short life span (median of 25 days in solid media),3 makes C. elegans an excellent model for the aging studies.4 Besides the extensive debates regarding the biological significance of the aging process,5 common sense suggests that life span of organisms should adequately suit their habitat, living conditions, social relations with piers and enemies, etc. End of life is characterized by various age-related changes that manifest the exhaustion of renewal capacity in tissues and failure to maintain the body homeostasis. Environmental cues and conditions, a well as dietary variations can greatly affect both average and maximal life span, and that is conveniently observed in the C. elegans model.4 The environmental hazards that can accelerate aging include excessive levels of reactive oxygen species (ROS). Indeed, many stresses and environmental insults result in increased levels of endogenous ROS that inflict damages to biological molecules, organelles, cells and extracellular matrices, and lead to a significantly shorter life. This is why the free-radical theory of aging,6,7 has inspired one of the most popular branches of aging studies during many years. However, more recent studies reveal that metabolic and dietary variations can also affect the lifespan. Different protocols of dietary restriction were found to increase substantially life span in animal model systems, including C. elegans .8-10
Genetic studies conducted in the C. elegans model have identified number of genes that positively or negatively affect lifespan of organisms, and the model is now extensively used for studying signaling pathways that are involved in the regulation of a lifespan.4 The lifespan-affecting genes act in several pathways that relate to metabolic regulation, such as the insulin-like growth factor receptor-1 (IGF-1) pathway that switches metabolism depending on the availability of carbohydrate nutrients,11 the mechanistic target of rapamycin (TOR) kinase pathway that keeps a balance between anabolic and catabolic processes in response to various nutrient and energy cues,12,13 the autophagy pathway that partially depends on the activity of TOR kinase complexes and is responsible for the timely utilization of a damaged or an excessive proteins or organelles and is important for the mobilization of internal nutrient resources during a starvation.14 There are also number of genes and processes that affect the pace of aging and lifespan in C. elegans as well as in mammals that include Sirtuins, members of the (NAD)-dependent protein deacetylase family, the AMPK-FOXO pathway activated by low energy levels,15 genes connected with epigenetic regulation16 and histone-modifying enzymes.17 Mitochondrial functions also affect the aging as they orchestrate and integrate metabolic processes, stress-induced responses and the production of ROS.18,19
Aging is a complex process, and many important connections between the identified genes and pathways are still unknown. In animals members of a small family of genes called sestrins (SESN1, SESN2 and SESN3),20-22 seem to have a connection with the aging process and lifespan regulation. The genes have differential regulation and selectively respond to stresses and influences through activation of p53, Nrf2, FOXO and HIF-1 transcription factors.23-28 SESN2 was found to have an antioxidant activity,29 that contributes to homeostatic regulation and protective functions of the p53 tumor suppressor gene.30,31 Sestrins were also found to be negative regulators of mTORC1 though their binding to AMPK and TSC2,32 and positive inducers of the autophagy.33 Recently sestrins were also found to serve as sensors of leucine levels on negative regulators of TOR complex 1 under conditions of a shortage of amino acids.34-36 All of the identified activities of sestrins may negatively affect the aging process, and indeed it was found that in Drosophila melanogaster model deficiency of dSesn gene results in a chronic activation of TOR, accumulation of ROS and premature manifestations of aging,37 and in C. elegans model deletion of cSesn gene was associated with a reduced lifespan.38
Here we describe some additional phenotypic characteristics of C. elegans that are deficient for the single cSesn gene that apparently relate to its activities that contribute to metabolic homeostasis and delay the aging processes.
Materials and Methods
Strains
The wild-type N2 and the RB2325 (genotype ok3157) with a 535 bp deletion in exon 3 of cSesn gene) strains of C. elegans were purchased in the Caenorhabditis Genetics Center (CGC) of University of Minnesota. The strain IE24589 deficient for cSesn gene expression due to the embedded MOS-1 transposon was gift by Prof. Yohann Duverger, University of Lyon, France.
Maintenance of nematodes
Maintenance of nematodes in solid agar medium was carried out according to the standard method.39 Sterilized agar containing 2% agarose in 0.3% sodium chloride, 1 mM magnesium sulfate, 1 mM calcium chloride, 0.25% 1 M phosphate buffer (108.3 g potassium phosphate, 35.6 g potassium di-phosphate dibasic, distilled water to a liter), 0.3% bactopeptone, and 0.05% cholesterol. A night culture of E. coli (strains OP50 or HT115) was applied to solidified agar beds in Petri dishes and the bacterial layer was grown overnight at 37°C. All experiments with nematodes were carried out at a temperature of 20°C.
Synchronous nematodes culture
To prepare a synchronous culture of nematodes, adult animals were treated with a solution containing 1% sodium hypochlorite and 0.5 M potassium hydroxide for 10 minutes. The released nematode eggs were washed twice in distilled water and incubated overnight at a temperature of 20°C in M9 medium (potassium phosphate, 6 g sodium phosphate dibasic, 5 g of sodium chloride, 1 ml of 1 M magnesium sulfate, distilled water to 1 liter). The next day, nematodes at the L1 stage were planted on Petri dishes containing bacterial food.
RNAi constructs
The RNA-interfering construct for cSesn gene was constructed as described previously.40 A construct expressing a cSesn cDNA fragment of 1434 bp was obtained by cloning into the pPD129.36 vector (Addgene pL4440) using XbaI and BamHI sites. The efficiency of RNA interference was verified by real-time PCR.
Pharyngeal pumping rate
The rate of contraction of the pharynx was calculated as described previously.41 The nematode, one at a time, was placed at room temperature on top of a standard agar medium with a bacterial layer at room temperature. Counting of contructions of the posterior pharynx was carried out during 1 minute using Nikon SMZ800 stereoscope under the magnification x210, every 4 days. Three independent experiments were conducted with 25 animals in each group.
Body size
Measurement of linear dimensions nematodes was carried out using the Keyence VHX 5000 microscope with the enclosed software package, daily during the first 8 days, then every second day up to Day 16, when nematodes stopped their growth. Two independent experiments were conducted with 200 animals in each group.
Brood Size
The nematodes in stage L4 were transferred one by one to a standard medium in 40 mm Petri dishes. The nematodes were then transferred daily to fresh dishes until the end of larval stages. The offspring were counted after reaching the larval stage L3-L4. Three independent experiments were conducted with 20 animals in each group.
Locomotory Activity
The locomotory activity of nematodes was evaluated as described earlier.42 The nematodes were divided into 4 groups, depending on the reaction to touch with a platinum wire tip: the A” group – the nematodes are active bending and moving without touching; the “B” group – the nematodes do not move without a touch or move chaotically; the “C” group – the nematodes move only the tail or head in response to a touch; the “D” group – the nematodes are dead. Three independent experiments were conducted with 100 animals in each group.
Statistical analysis
Data was processed using STATISTICA software package (http://www.statistica.io). The reliability of the difference between the two mean values was estimated using Student’s t-test; more than two independent samples were compared using the ANOVA dispersion analysis. In all graphs: p-value: * = <0,05, ** = <0,01, *** = <0,001.
Results and Discussion
We decided to compare phenotypes of three different populations of C. elegans that have deficiency in the cSesn gene with the standard wild-type strain N2. The cSesn deficient populations included the RB2325 strain that have a 525 bp deletion in exon 3 of cSesn gene (ok3157 genotype), IE24489 strain with insertion of MOS-1 transposon to exon 2 (ttTi24589 genotype) and the wild-type strain N2 in which cSesn gene expression was compromised by RNA interference (the worms were fed with bacteria carrying cDNA-expressing plasmid for the cSesn transcript).
To reveal phenotype changes connected with the deficiency of cSesn gene we have chosen the parameters that are known to change during the aging process. The age-related changes in C. elegans include body size,42 fertility, or number of offspring,43 changes that characterize the age-related decay in the muscle tissue – the pumping rate of the C. elegans pharynx,44 and changes in the locomotory activity.45
Body Size
During the first two weeks from the young adult stage of C. elegans (L4) the linear body size increases by 70%, in width – by 30%, and in volume – by 280%.46,47 Functions of genes belonging to different signaling pathways, especially in the IGF/insulin circuit, simultaneously affect both the body size and the lifespan, which is observed in nematodes with mutations in appropriate genes. It was observed that there is a correlation between a large body size and a short lifespan.48 By comparing the dynamics of body size growth between the wild-type N2 and cSesn-deficient strains of C. elegans we noticed substantial differences. All three groups og cSesn-deficient worms has demonstrated similar growth rates and maximal body sizes. Under the growth conditions by Day 8 the cSesn-deficient strains reached sizes that exceed the sizes of the wild-type N2 worms by 47.59% (Fig. 1). While the N2 nematodes stoped growing by Day 5, the cSesn-deficient animals continued the growth up to Day 8.
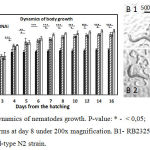 |
Figure 1: A. Dynamics of nematodes growth. P-value: * – < 0,05; ** – < 0,01; *** – < 0,001. B. Worms at day 8 under 200x magnification. B1- RB2325 cSesn knockout strain, B2 – wild-type N2 strain.
|
Fertility
The reproductive function and fertility decrease with age in almost all animals. In humans, the old age of a mother is directly linked with a high incidence of genetic abnormalities in the newborn, which is caused by aged oocytes.49 A similar trend is observed in nematodes.50 On the other hand, some studies indicate an acceleration in aging, depending on the number of offspring, due to the fact that the resources spent on producing offspring become inaccessible to maintain homeostasis.51 Reduction of reproductive activity can also be an adaptive response to a decrease in metabolic processes in general. Nematode hermaphrodite is capable of self-fertilization. On average, one animal gives about 250-300 offspring. A general decrease in the number of offspring is shown for mutants for a number of aging-related genes.43 We checked the number of offspring in mutants and in wild type N2 nematodes. A single wild-type C. elegans is known to be capable of producing from 200 to 330 ± 40 offspring.52,53 In our experiments, the wild type gave approximately 230 offspring from one worm, which is by 77.45 % exceeds the number of the offspring (140 ± 20) produced by a single worm from the cSesn-deficient populations (Fig. 2).
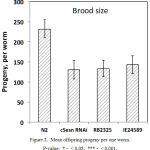 |
Figure 2: Mean offspring progeny per one worm. P-value: * – < 0,05; *** – < 0,001.
|
Decline in the Muscles
It is known that signaling pathways related to redox generation and redox response are involved in myogenesis, while elevated levels of ROS can lead to a damage to myofibrils and death of myocytes.54 Muscle tissue and its functions are affected during aging in C. elegans. These parameters are known to decline during the aging process.
In mammals the sestrin genes are involved in the redox regulation and may act as antioxidants suggesting that in C. elegans cSesn gene could affect homeostasis in muscles. We monitored the dynamics of age-related changes by measuring rates of pharyngeal contraction (or pumping rate) and locomotory activity. C. elegans use pharynx, a neuromuscular organ, to ingest the food by pushing it into the digestive tract. The rate of pharyngeal contractions depends on the quantity and quality of food,55 and on the age of the nematode.56 The age-related decline in the rate of pharyngeal pumping rate is caused by the accumulation of degenerative changes in the muscles. 56 We found that in the cSesn-deficient worms a faster decline in the pharyngeal pumping rate is observer as compared with the wild-type N2 C. elegans strain (Fig.3), which can be explained by a more rapid degeneration of the pharyngeal apparatus.
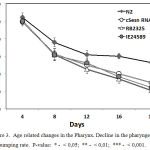 |
Figure 3: Age related changes in the Pharynx. Decline in the pharyngeal pumping rate. P-value: * – < 0,05; ** – < 0,01; *** – < 0,001.
|
We also tested the dynamics of the age-related decline of the locomotory activity, which can be regarded as an additional parameter related to the aging process in C. elegans. During the course of life, most animals experience progressive decline in locomotory activity and coordination of movements, which is due to reduced strength and degeneration of muscles. These changes can be studied by using C. elegans, the simple model organism with well-known and easily modifiable genotype.57 Young adults of C. elegans demonstrate well-coordinated energetic sinusoidal movements of the body, which gradually slow down, reduce coordination and eventually cease completely.58 When describing these changes, three locomotory activity classes are determined. The nematodes pass from the first (rhythmic sinusoidal movements) to the second (less active and uncoordinated) and end down to the third (no regular, just occasional movements, or separate movements of the tail or head in response to a touch). 42,58 The age-related changes in locomotory activities has been extensively described in a number of studies 42,58-60 and is now widely used as one of the markers of age-related changes in the nematode. Our measurements with three strains of C. elegans with deficient expression of cSesn have demonstrated a significantly more rapid decline in the frequency, rhythm and coordination of mutant worms as compared with the wild-type N2 strain (Fig. 4). The result indicates that cSesn is required for maintaining the normal homeostasis of the entire muscular mass in the nematode body.
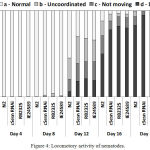 |
Figure 4: Locomotory activity of nematodes.
|
The nematodes were divided into four groups based on their locomotory activity: Group A – normal locomotion; Group B – uncoordinated movements; Group C – worms move only head or tail, in response to a touching; Group D – dead worms. The bars indicate the proportion of animals of each group at the designated day.
The revealed accelerated age-related changes in the populations of C. elegans with a compromised expression of cSesn gene could be explained by an involvement of cSesn in metabolic and redox regulation. The increase in the body size could be explained by the participation of cSesn in the IGF1-TOR signaling pathway, while the rapid decrease of pharyngeal pumping and the decline in in locomotory activity could be due to a faster accumulation of muscle damage produced by excessive ROS. The reproductive functions nematodes are regulated partly by the IGF1 and TGF-beta signaling pathways,61,62 as in the mammals a deficiency in Sestrins can induce activation of the TGF-beta signaling.63,64 Therefore, the decreased number of offspring in the mutant worms could be explained by these changes. However, more studies are required to reveal mechanisms responsible for the observed phenotype changes in C. elegans.
Conclusion
In conclusion, the results obtained here indicate that the cSesn gene plays an important role in the processes of age-related decline in the nematode C. elegans model. The deficiency in cSesn results in an increase body size and an extended period of growth. There was also reduced number of offspring and an accelerated decline in pharyngeal pumping rate and locomotory activity. The changes are well explained by the antioxidant and mTOR-inhibiting functions of Sestrins revealed in mammals. However, more studies are required to identify particular mechanisms that act behind the observed phenotype changes.
Acknowledgements
The study was supported by the Program of fundamental research for state academies for 2013-2020 years (number 01201363823) and by grants from the Russian Science Foundation 14-50-00060 (to P.M.C.) and number 17-14-01420 ( to A.V.B.).
References
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71-94.
- Harris T.W, Antoshechkin I, Bieri T, Blasiar D, Chan J, Chen W.J, De L.C.N, Davis P, Duesbury M, Fang R, Fernandes J, Han M, Kishore R, Lee R, Muller H.M, Nakamura C, Ozersky P, Petcherski A, Rangarajan A, Rogers A, Schindelman G, Schwarz E.M, Tuli M.A, Van Auken K, Wang D, Wang X, Williams G, Yook K, Durbin R, Stein L.D, Spieth J, Sternberg P.W. WormBase: a comprehensive resource for nematode research. Nucleic Acids Res. 2010;38:463-467.
CrossRef - Sutphin G.L ,Kaeberlein M. Measuring Caenorhabditis elegans life span on solid media. J Vis Exp. 2009.
CrossRef - Uno M, Nishida E. Lifespan-regulating genes in C. elegans. NPJ aging and mechanisms of disease. 2016;2:10-160.
- Weinert B.T,Timiras P.S. Invited review: Theories of aging. J Appl Physiol. 2003;95:1706-1716.
CrossRef - Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298-300.
CrossRef - Beckman K.B, Ames B.N. The free radical theory of aging matures. Physiol Rev. 1998;78:547-581.
CrossRef - Masoro E.J. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913-922.
CrossRef - Greer E.L, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113-127.
CrossRef - Brunet A. A CRTCal link between energy and life span. Cell Metab. 2011;13:358-360.
CrossRef - Kenyon C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann N Y Acad Sci. 2010;1204:156-162.
CrossRef - Wullschleger S, Loewith R, Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471-484.
CrossRef - Antikainen H, Driscoll M, Haspel G, Dobrowolski R. TOR-mediated regulation of metabolism in aging. Aging Cell. 2017;16:1219-1233.
CrossRef - Lapierre L.R, Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol Metab. 2012;23:637-644.
CrossRef - Greer E.L, Dowlatshahi D, Banko M.R, Villen J, Hoang K, Blanchard D, Gygi S.P, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646-1656.
CrossRef - Greer E.L, Maures T.J, Ucar D, Hauswirth A.G, Mancini E, Lim J.P, Benayoun B.A, Shi Y, Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;2011:1038.
- Greer E.L, Maures T.J, Hauswirth A.G, Green E.M, Leeman D.S, Maro G.S, Han S, Banko M.R, Gozani O. Brunet A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383-387.
CrossRef - Dillin A, Hsu A.L, Arantes-O.N, Lehrer-G.J, Hsin H, Fraser A.G, Kamath R.S, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science.
- Van R.J.M, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS genetics. 2009;5:e1000361.
CrossRef - Velasco M.S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127-137.
CrossRef - Budanov A.V, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, Skaliter R, Gudkov A.V, Chumakov P.M, Feinstein E. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017-6031.
CrossRef - Nogueira V, Park Y, Chen C.C, Xu P.Z, Chen M.L, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer cell. 2008;14:458-470.
CrossRef - Chumakov P.M. Versatile functions of p53 protein in multicellular organisms. Biochemistry (Mosc). 2007;72:1399-1421.
CrossRef - Kopnin P.B, Agapova L.S, Kopnin B.P, Chumakov P.M. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67: 4671-4678.
CrossRef - Chen C.C, Jeon S.M, Bhaskar P.T, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
CrossRef - Olson N, Hristova M, Heintz N.H, Lounsbury K.M, van d.V.A. Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2011;301:993-1002.
CrossRef - Shin B.Y, Jin S.H, Cho I.J, Ki S.H. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med. 2012;53:834-841.
CrossRef - Shi X, Doycheva D.M, Xu L, Tang J, Yan M, Zhang J.H. Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiology of disease. 2016;95:111-121.
CrossRef - Budanov A.V, Sablina A.A, Feinstein E, Koonin E.V, Chumakov P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596-600.
CrossRef - Sablina A .A, Budanov A.V, Ilyinskaya G.V, Agapova L.S, Kravchenko J.E, Chumakov P.M. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306-1313.
CrossRef - Olovnikov I.A, Kravchenko J.E, Chumakov P.M. Homeostatic functions of the p53 tumor suppressor: regulation of energy metabolism and antioxidant defense. Seminars in cancer biology. 2009;19:32-41.
CrossRef - Budanov A.V,Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451-460.
CrossRef - Maiuri M.C, Malik S.A, Morselli E, Kepp O, Criollo A, Mouchel P.L, Carnuccio R, Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571-1576.
CrossRef - Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim Y.C, Akopiants K, Guan K.L, Karin M, Budanov A.V. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell reports. 2014;9:1281-1291.
CrossRef - Budanov A.V. SESTRINs regulate mTORC1 via RRAGs: The riddle of GATOR. Molecular & cellular oncology. 2015;2(9):97-113.
CrossRef - Kim J.S, Ro S.H, Kim M, Park H.W, Semple I.A, Park H, Cho U.S, Wang W, Guan K.L, Karin M, Lee J.H. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific reports. 2015;5:9502.
CrossRef - Lee J.H, Budanov A.V, Park E.J, Birse R, Kim T.E, Perkins G.A, Ocorr K, Ellisman M.H, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223-1228.
CrossRef - Yang Y.L, Loh K.S, Liou B.Y, Chu I.H, Kuo C.J, Chen H.D, Chen C.S. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Exp Gerontol. 2013;11:00008-00009.
CrossRef - Stiernagle T. Maintenance of C. elegans. WormBook. 2006;11:1-11.
CrossRef - Timmons L, Court D.L, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103-112.
- Raizen D, Song B.m, Trojanowski N, You Y.J. Methods for measuring pharyngeal behaviors. 2005.
- Herndon L.A, Schmeissner P.J, Dudaronek J.M, Brown P.A, Listner K.M, Sakano Y, Paupard M.C, Hall D.H, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C-elegans. Nature. 2002;419:808-814.
CrossRef - Hughes S.E, Evason K, Xiong C.J, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLOS Genetics. 2007;3:254-265.
CrossRef - Chow D.K, Glenn C.F, Johnston J.L, Goldberg I.G, Wolkow C.A. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Experimental Gerontology. 2006;41:252-260.
CrossRef - Kirkwood T.B.L,Finch C.E. Ageing – The old worm turns more slowly. Nature. 2002;419:794-795.
CrossRef - Bolanowski M.A, Russell R.L, Jacobson L.A. Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech Ageing Dev. 1981;15:279-295.
CrossRef - Croll N.A, Smith J.M, Zuckerman B.M. The aging process of the nematode Caenorhabditis elegans in bacterial and axenic culture. Exp Aging Res. 1977;3:175-189.
CrossRef - McCulloch D,Gems D. Body size, insulin/IGF signaling and aging in the nematode Caenorhabditis elegans. Exp Gerontol. 2003;38:129-136.
CrossRef - Hartge P. Genetics of reproductive lifespan.//. (Nat Genet. 2009;41(6):637-8. doi: 10.1038/ng0609-637.).
CrossRef - Luo S, Kleemann G.A, Ashraf J.M, Shaw W.M, Murphy C.T. TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell. 2010;143:299-312.
CrossRef - Kirkwood T.B. Evolution of ageing. Nature. 1977;270:301-304.
CrossRef - Ward S,Carrel J.S. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Developmental biology. 1979;73:304-321.
CrossRef - Pickett C.L, Dietrich N, Chen J, Xiong C, Kornfeld K. Mated Progeny Production Is a Biomarker of Aging in Caenorhabditis elegans. G3: Genes|Genomes|Genetics. 2013;3:2219-2232.
CrossRef - Bakkar N,Guttridge D.C. NF-κB signaling: a tale of two pathways in skeletal myogenesis. 2010;90:495-511.
- Avery L,Shtonda B.B. Food transport in the C. elegans pharynx. J Exp Biol. 2003;206:2441-2457.
CrossRef - Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8084-8089.
CrossRef - Guarente L,Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255-262.
CrossRef - Hosono R, Sato Y, Aizawa S.I, Mitsui Y. Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol. 1980;15:285-289.
CrossRef - Newell S.B.L, Cypser J.R, Kechris K, Kitzenberg D.A, Tedesco P.M, Johnson T.E. Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway. Aging Cell. 2018.17:7.
- Kim J.S, Kim S.H, Park S.K. Selenocysteine modulates resistance to environmental stress and confers anti-aging effects in C. elegans. Clinics. 2017;72:491-498.
CrossRef - Wang M.C, Oakley H.D, Carr C.E, Sowa J.N, Ruvkun G. Gene Pathways That Delay Caenorhabditis elegans Reproductive Senescence. PLOS Genetics. 2014;10(100):47-52.
CrossRef - Luo S, Shaw W.M, Ashraf J, Murphy C.T. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009;5:24.
CrossRef - Wempe F, De Z.S, Koli K, Bangsow T, Parajuli N, Dumitrascu R, Sterner K. A, Weissmann N, Keski O.J, von M. H. Inactivation of sestrin 2 induces TGF-beta signaling and partially rescues pulmonary emphysema in a mouse model of COPD. Dis Model Mech. 2010;3:246-253.
CrossRef - Hay N. Interplay between FOXO, TOR, and Akt. Biochimica et biophysica acta. 2011;18(13):1965-1970.
CrossRef







