Vishnu T. S and Palaniswamy M.
Department of Microbiology, Karpagam Academy of Higher Education, Coimbatore 641021, Tamil Nadu, India.
Corresponding Author E-mail: tsvishnu1988@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1423
Abstract
Violacein was isolated from Chromobacterium vaccinii CV5, a Gram negative bacterium collected from the Well water near a paddy field- Nedumangadu, Kerala, India. In the current antioxidant study, the activities of violacein were analyzed by different scavenging assay. In vitro antioxidant activity of violacein pigment was studied with the antioxidant, hydrogen peroxide DPPH, nitric oxide, hydroxyl and superoxide. The results suggest that the violacein possess potent activity of antioxidant against DPPH, superoxide, nitricoxide, hydrogen peroxide and hydroxyl with IC50 values of 297.88 µg/mL, 312.89 µg/mL, 410.17 µg/mL, 296.74 µg/mL and 292.74 µg/mL respectively. The activity was comparable with the standard L-ascorbic acid. Natural compounds are very much used for the treatment of chronic diseases because of their effectiveness and have less harmful as compared to synthetic and artificial drugs. The present study’s shows the ability of the pigments as a source of natural antioxidants which has various applications in pharmaceutical industries.
Keywords
Antioxidant; Chromobacterium; DPPH; Pigment; Violacein
Download this article as:| Copy the following to cite this article: Vishnu T. S, Palaniswamy M. Systematic Approach on Evaluating the In Vitro Antioxidant Activity of Violacein; Novel Isolate Chromobacterium Vaccinii CV5. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Vishnu T. S, Palaniswamy M. Systematic Approach on Evaluating the In Vitro Antioxidant Activity of Violacein; Novel Isolate Chromobacterium Vaccinii CV5. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20361 |
Introduction
Now a day in many areas such as medicine, food, ink, paper, textiles, aquaculture, animal feed and food natural colorants are used to a large extent. Violacein, a versatile colored compound commonly produced from Chromobacterium sp that has wide biological properties and its increasing interest, has gained importance in pharmaceutical and industrial markets such as in cosmetics textiles.
It is an indole derived violet colour pigment produced by other bacterial strains such as Janthinobacterium lividum, Chromobacterium lividum and Pseudoalteromonas luteoviolacea. Chromobacterium violaceum is one of the common bacterium studied in the production of violacein, other than C. violaceum many other bacterium are able to produce violacein in variable yields and productivity .Violacein production is the one of the main characteristics of Chromobacterium sp. The secondary metabolites produce under aerobic condition by bacteria is of great importance for pharmacological applications.1
Violacein isolation and biochemical characterization began in 1958 and it was finally spectroscopically analyzed in 1984.2 Violacein has various multiple biological activities like antibiotic,3 trypanocide,4 antitumoral,5-8 antiviral,9 genotoxic10 and antioxidant11 properties.
Now a day most research works are concentrated on natural compounds that exhibit various biological application and pharmaceutical industries. Most of them possess potent antioxidant activity. Antioxidant has the capacity to protect the cell and cell damage because of oxidative stress. Oxidative stress play an important role in human diseases such as tumors ,malignancy, emphyssema, liver cirhosis, atheroscclerosis and many types of arthritis all are clinically correlated with oxidative damages.12 The study of violacein as an antioxidant has been analyzed in many models, as a scavenge the 2, 2-diphenyl-1-picrylhydrozyl (DPPH) radical, nitric oxides, inhibits lipid peroxidation and hydroxyl free radicals.13 In vitro cytotoxic effect of violacein produced by novel isolate Chromobacterium vaccinii CV5 against the cervical and lung cancer cell was studied14 in the earlier work. This study analyses the invitro antioxidant activity of violacein produced by the novel isolate Chromobacterium vaccinii CV5 is reported.
Materials and Methods
Microorganisms
The Chromobacterium vaccinii CV5 isolated from the well water identified by morphologically, biochemically and genetically was used in the present study.15
Pigment Production and Purification
The production of crude violacein was measured by using a 500mL flask containing 200mL of nutrient broth. Fermentations were done at 37°C for 48 h with an inoculum volume of 3% (v/v) 24 h old culture (OD 660 approximately 1).16 After 48 h of incubation period broth was taken for pigment extraction. The partially purified compound was use for the further studies.
DPPH (1, 1-Diphenly2picryl-hydrozil) Free Radical Scavenging Activity
The in vitro antioxidant activities of violacein were determined by DPPH (2, 2-diphenyl.1- icrylhydrazyl) radical scavenging activities. 300µL of different concentrations (100, 200, 300, 400 and 500mg/mL) of sample was taken .To this add 100µL and make up to 400µL of total volume with distilled water. Add 600µL of 100.m DPPH solution make up in methanol. Incubate the reactions mixtures for 20min in darken condition. The absorbant reading was appropriated and taken at 517nm. Antioxidant capacity of violacein was determined by the decrease in absorbance readings. L-ascorbic acid was kept as a standard.17
Nitric Oxide Scavenging Assay
Nitric oxide produced from sodium nitroprusside was analyzed and measured by Griees reaction.18 About 100, 200, 300, 400 and 500µg/mL concentrations of crude pigment extracts was previously dissolved in DMSO was make up to 3mL reaction mixture using sodium nitroprusside (10mm) in phosphate buffer saline. Besides that ascorbic acid (standardized compounds) were taken in additional tube. Incubate the mixtures at 25°C for about 150min, then coming to the removal of 1.5mL of reaction mixture. After incubation, 1.5mL of Griees reagents was supplemented in the each tube over and above control also. The reading of absorbance was measured at 546nm on UV-Visible spectrophotometer using methanol as blank.18
Superoxide Scavenging Activity
Superoxide anion scavenging activity was Measurements by the Nishimiki et al19 method .1mL of nitrobluetetrazolium (NBT 156mM in 10mM phosphate buffer, pH 7.4) solutions, 1mL NADH solutions (468mM in 100 mM phosphate buffers, pH7.4) and 100uL of violacein extract varying concentration (100, 200, 300, 400 and 500µg/mL) in the DMSO are taken in each tubes. To the reaction mixtures add 100 mL of phenazinemethosulphate (PMS) solution (60mM PMS in 100mM phosphate buffers, pH 7.4) to the mixture. Incubate the reaction mixture at 25°C for 5min and the absorbant reading was taken at 560nm against the blank sample. Decrease in absorbance of the reaction mixture indicates the increased superoxide anion scavenging activities. L-Ascorbic acid was taken as the standards.
Hydroxyl Scavenging Assay
Hydroxyl radical scavenging activity of the pigment was estimated by method of Klein et al.20 OH radicals scavenging activities of pigment was analyzed by using 100, 200, 300,400 and 500μg/mL of the pigment extract was taken in different test tube. 1mL of iron EDTA solution (0.13% ferrous ammonium sulfate and 0.26%EDTA), 0.5mL of 0.018% EDTA and 1mL of 0.85% (v/v) DMSO (in 0.1 M phosphate buffers, pH7.4) was added and follows by 0.5 mL of 0.22% (w/v) L- ascorbic acid. The tube are tightly capped and incubate the tubes in a water bath at 85 ºC for 15min. after incubation period, uncap the test tube and add ice-cold trichloroaceticacid (17.5% w/v) to each tubes immediately. To that mixture add 3mL of Nash reagent (7.5g of ammonium acetate, 300μL glacial aceticacid and 200μL acetylacetone was mixed and make up to 100mL with distilled water) to all the tubes and incubate the tubes in at room temperature for 15min. Absorbance of mixture was taken at 412 nm. Percentage hydroxyl radical scavenging activity (HRSA %) was calculated by following formula: HRSA % = [(Abs (control) – Abs (Sample)/Abs (Control)] x 100.
Hydrogen Peroxide Scavenging Assay
The hydrogen peroxides (H2O2) activities were determined by mean of the decrease in the absorbance at 230nm spectrophotometer. The antioxidant activity of the violacein to scavenge hydrogen peroxide was measured based on Ruch et al.21 Hydrogen peroxide (2mmol/L) was making up in phosphate buffer (pH 7.4). The concentration of hydrogen peroxide activities was measured taking absorption at 230 nm using a spectrophotometer. 100, 200, 300, 400 and 500 mg/mL of pigment extracts in the DMSO were added to 600uL hydrogen peroxide solution. The Absorbances of hydrogen peroxide was measured at 230nm and after 10min measure blank solutions having DMSO without the hydrogen peroxide.
Total Reducing Assay
Total reducing activity was measured based upon the method of Lin et al22 Different concentrations (100, 200, 300, 400 and 500mg/mL) of violacein extract (0.25mL) was mixed to equal volumes of 200mM sodium phosphate buffers (pH6.6) and 0.25mL of 1% potassium ferric cyanide. Incubate the reaction mixtures at 50°C for 20min.Add 250uL of 10% trichloro acetic acid to the reaction tubes to stop the reactions, again centrifuge the reaction mixture at 3000.rpm for 10min. 500Ul of supernatants were mixed with 400uL of deionized water and 100uL of 0.1% ferric chloride solution, kept for 10 min. Absorbance was measured at 700 nm and higher absorbant readings indicate higher reducing capacities.
Result and Discussion
Among the many of them are responsible for many clinical syndromes likes neurodegenerative diseases, tumors, AIDS and many auto immune disorders scavenging activity of these compounds are exploited to eradicate these diseases. In the view of the up surging interest in the health benefits of the microbial pigment, the antioxidant effects of partially purified violacein pigment from C. vaccinii CV5 is evaluated. Ascorbic acid act by breaking chain that in turn will decrease the formations of free radicals .in this process they form intracellular substances throughout the body including tooth dentines bone matrix and collagen.23,24 L-Ascorbic acid is used as the standard for the whole antioxidant study. Konzen et al [11] studied the antioxidant activities of violacein. In their study, violacein extracts react with DPPH radicals (IC50=30.lM), nitricoxide (IC50=21.lM), superoxide free radicals (IC50 = 125.lM) and the hydroxyl radicals EPR signals was decreased.
DPPH Radical Scavenging Assay
The DPPH scavenging is rapid cost effective and accurate test to analyze the in vitro antioxidant capacity of the compound.25,26 The figure.1 shows the DPPH free scavenging activities of violacein pigment. Pigment exhibits a dose dependent higher free radicals scavenging capacity with an IC50 values of 297.88µg/mL. However the activity is lesser than that of L-ascorbic acid (IC50= 236.74µg/mL). The current study revealed the antioxidant activity of violacein from C.vaccinii CV5.Violacein could act as a scavengers or free radical inhibitor so that it act as primary antioxidant, which can help in slow down or preventing the progress of different clinical disorders to oxidative stress and bacterial contamination.
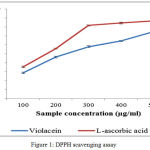 |
Figure 1: DPPH scavenging assay
|
Nitric Oxide Scavenging Assay
Free radical and active oxygen species which is produced in the body are responsible for various pathological and clinical disorders. Nitric oxide is also involved in many other inflammations like tumors and many auto immune syndromes. The frequent cause of oxidation of damages is the oxidations of thyrosine residues of proteins, lipid peroxidation, and degradation DNA degradation and oligo nucleosomal fragment. Nitric oxide or reactive nitrogen species that are formed during the serious of reaction with oxygen or with superoxides such as NO2, N2O4, N3O4, nitrates and nitrites are very toxic. These toxic molecules may cause changes in the structure and function of many intra and extra cellular components. Any compounds, naturals or synthetics, with antioxidant activity might contribute towards the total or partial alleviation of those damages.27 The figure.2 illustrate there is a remarkable decrease in the free radical by the scavenging action of violacein and L-ascorbic acid. The IC50 values obtained are 256.4µg/mL for L-ascorbic acid and 312.89µg/mL for violacein respectively.
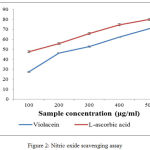 |
Figure 2: Nitric oxide scavenging assay |
Superoxide Scavenging Assay
The results obtained from the study emphasize the scavenging capacity of violacein to annihilate the superoxide anion produced in PMS-NADPH-NBT systems (Fig.3). The percentages of scavenging activities are increased when the concentration of the sample was increased. L-ascorbic acid shows IC50 value of 254.4µg/mL. The IC50 value of violacein is 410.17µg/mL. Superoxide anion is the reduced form of molecular oxygen that implicates the initiation of oxidation reactions associate with ageing process.28 It is also involved in several other physiological process, due to its conversion into related reactive species substances mostly hydroxyl radicals that initiates peroxidations of lipids. Some flavor enzymes like xanthine oxidase produce super anion free radicals which will convert the hypoxanthine and other similar compounds to uric acid.29 Other reactive oxygen species which includes singlet oxygen, hydroxyl radical and hydrogen peroxides, which induce oxidation and causes damages in lipids, proteins and nucleic acids.30,31
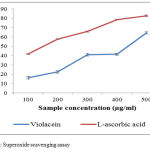 |
Figure 3: Superoxide scavenging assay
|
Hydroxyl Scavenging Assay
The scavenging ability of pigments towards hydroxyl radicals, shown in Figure 4, is dose dependent, reaching 85.7 and 73.970% at 1000µg/mL for C.vaccinii CV5 and ascorbic acid respectively. The IC50 value for violacein is 296.74µg/mL. Ascorbic acid is taken as standard control which was highly effective in quenching of OH radical (IC50=247.15µg/mL). Hydroxyl radicals are a highly reactive species produced in the biological system and have capacity to induce damages in almost all intra cellular organelles in living cell.32 The free radicals has the ability to combine with nucleotides in DNAs and induces damages in strands induces carcinogenic to cells, mutation and cell cytotoxicity.33
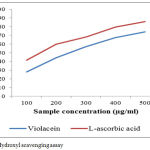 |
Figure 4: Hydroxyl scavenging assay
|
Hydrogen Peroxide Scavenging Assay
The Hydrogen peroxide scavenging activities of violacein sample to effectively determine by the method of Ruch et al,21 their activity is compared with the L-ascorbic acid. The pigment exhibits a very good scavenging activity on hydrogen peroxide on increasing the concentration of violacein extracts (Figure5). IC50 value of scavenging of H2O2 for violacein is 292.74µg/mL and L-ascorbic acid are 239.46µg/mL respectively. Mostly Hydrogen peroxides are non-toxic and non-reactive, but sometimes Hydrogen peroxide is converted into hydroxyl molecules insides the cells and react with biomolecules to form toxic substances which causes cell death and tissue damages.34
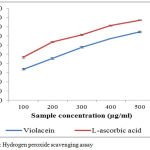 |
Figure 5: Hydrogen peroxide scavenging assay
|
Reducing Capacity
The total reducing activity of a violacein shows potency to serve as a strong antioxidant agent .Many recent studies states that bioactive compounds have the capacity to donate electrons which will indicates the antioxidant activity.35,36 The ferrous form of iron fe3+ was reduced ferric irons was determined by the colour intensity of blue-greenish solutions and the absorbant readings is measured at 700nm.The present study states that change in colour of test solutions from yellow to some distinct shades of greenish and blue. The colour change determines the potency of violacein extracts as a powerful antioxidant molecule. Reducing activity of a compound determines the index of potentiality of antioxidant capacity.37 The pigment shows a significant effect concentrations are increased (figure: 6)
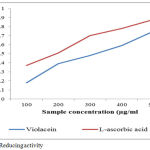 |
Figure 6: Reducing activity
|
Conclusion
The present study describes one of the promising natural microbial colorants, violacein produced by Chromobacterium vaccinii CV5 which can be used as a novel source of antioxidant agents. Under in vitro assay condition pigment efficiently scavenged free radicals. Thus the violacein pigment may be potential source for use in food and pharmaceutical applications. Since none had attempted so far, current study would be the first report for determining the better antioxidant activity of the novel isolate Chromobacterium vaccinii CV5. Thus, the further study was to purify the intracellular violet pigment and to characterize the properties which can contribute to a variety of applications in different field of pharmaceutical industries.
Acknowledgements
This works was not supported by any research grants
Conflicts of Interest
There is no conflict of interest.
References
- Ballantine J.A, Beer R.J, Crutchkey D.J, Dodd G.M. and Palmer D.R. The synthesis of violacein and related compounds. Chem. Soc., 1958;1:232-233.
- Lattasch H, Ronald H.T. and Philip J.C. Spectroscopic properties of violacein and related compounds: crystal structure of tetraethyl-violacein. Chem. Soc. Perkin. Trans., 1984; 2: 1331-1339.
CrossRef - De Souza A.O, Aily D.C.G, Sato D.N. and Durán N. In vitro activity of violacein against Mycobacterium tuberculosis Rev. Inst. Adolfo. Lutz., 1999; 58: 59-62.
- Duran N, Antonio R.V, Haun M. and Pilli R.A. Biosynthesis of trypanocide by Chromobacterium violaceum. J. Microbio.l Biotechnol., 1994; 10: 686–690.
CrossRef - Melo P.S, Justo G.Z, de Azevedo M.B.M, Duran N. and Haun M. Violacein and its 𝛽-cyclodextrin complexes induce apoptosis and differentiation inHL60 cells. , 2003; 186: 217-225.
- Ferreira C.V, Bos C.L, Versteeg H.H, Justo G.Z, Duran N. and Peppelenbosch M.P. Molecular mechanism of violacein mediated human leukemia cell death. Blood., 2004; 104: 1459–1464.
CrossRef - Saraiva V.S, Marshall J, Cools-Lartigue J. and BurnierJr M.N. Cytotoxic effects of violacein in human uvealmelanoma cell lines. Research. 2004; 4: 421–424.
- Kodach L.L, Bos C.L, Duran N, Peppelenbosch M.K, Ferreira C.V, Hardwick J.C.H. Violacein synergistically increases 5-fluorouracil cytotoxicity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogen., 2006; 27: 508-516.
CrossRef - Andrighetti-Frohner C.R, Kratz J.M, Antonio R.V, Creczynski-Pasa T.B, Barardi C.R.M, Simoes C.M.O. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violacein. Inst. Oswaldo. Cruz., 2003; 98: 843-848.
CrossRef - Andrighetti-Frohner C.R, Antonio R.V, Creczynski-Pasa T.B, Barardi C.R.M, Simoes C.M.O. In vitro testing for genotoxicity of violacein assessed by Comet and Micronucleus assays. Res., 2006; 603: 97-103.
- Konzen M, Marco D.D, Clarissa A.S. Cordova, Tiago O. Vieira, Regina V. Antonioc and Tania B. Creczynski-Pasaa. Antioxidant properties of violacein: Possible relation on its biological function. Bioorg. Med. Chem., 2006; 14: 8307–8313.
CrossRef - Nithiya S, Karthik N, Jayabharathi J. In vitro antioxidant activity of hindered piperidone derivatives. Intl J. Pharm. Pharmsci., 2011; 3: 254-256.
- de Sousa Leal A.M, Freires de Queiroz J.D, Batistuzzo de Medeiros S.R, de Souza Lima T.K. and Agnez-Lima LF. Violacein induces cell death by triggering mitchonral membrane hyperpolarization in vitro. BMC Microbiol., 2015; 15:115.
CrossRef - Vishnu T.S. and Palaniswamy M. Evaluation of in vitro cytotoxic effect of violacein produced by novel isolate Chromobacterium vaccinii CV5 against the cervical and lung cancer cell. Asian. J. Phar. Clin. Res., 2017:10(10):227-229.
CrossRef - Vishnu T.S. and Palaniswamy M. Isolation and identification of Chromobacterium sp from different ecosystems. J. Phar. Clin. Res., 2016; 9(3): 253-257.
- Vishnu T.S. and Palaniswamy M. Impact of various fermentation conditions on the production of violacein by the novel isolate Chromobacterium vaccinii Int. J. Pharm. Bio. Sci., 2017b;8(3):514-522.
- Srinivas S. and Prakash V. Effect of co-solvents on the stabilization of biological active peptides from bovine milk alpha casein. Pep. Lett., 2008; 15:371–374.
CrossRef - Green L.C, Wagner D.A, Glogowski J, Skipper P.L, Wishnok J.K. and Tannenbaum S.R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Biochem., 1982; 126: 131-138.
- Nishimiki M, Rao N.A. and Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biophys. Res. Comm., 1972; 46:849-853.
CrossRef - Klein S.M, Cohen G. and Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical generating system. Biochemist., 1991; 20: 6006-601.
CrossRef - Ruch R.J, Cheng S.J. and Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogen., 1989; 10: 1003-1008.
CrossRef - Lin E.S, Yang C.T, Chou H.J. and Chang T.T. Screening of antioxidant activities by the edible Basidiomycete Antrodia cinnamomea strains in submerged culture. J Food Biochem., 2010; 34: 1141-1156.
CrossRef - Beyer R.E. The role of ascorbate in antioxidant protection of biomembranes: interaction with vit-E and coenzyme. Q. J. Bioen. Biomemb., 1994; 24: 349-358.
CrossRef - Aqil F, Ahmed I, Mehmood Z. and Turk. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. J. Biol., 2006; 30:177-183.
- Koleva I.I, Van Beek T.A, Linseen J.P.H, de Groot A. and Evstatieva L.N. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal., 2002; 13:8- 17
CrossRef - Suresh P.K, Sucheta S, Sudarshana V.D, Selvamani P. and Latha S. Antioxidant activity in some selected Indian medicinal plants. .J Biotechnol., 2008; 7: 1826-1828.
- Sanja S.D, Sheth N.R, Patel N.K, Dhaval P. and Biraju Patel. Characterization and evaluation of antioxidant activity Of Portulacaoleracea J. Pharm. Pharmaceut. Sci., 2009;1: 74-84.
- Cotelle N, Bemier J.L, Catteau J.P, Pommery J, Wallet J.C, Gaydou E.M. Antioxidant properties of hydroxy flavones. Free. Radic. Biol. Med., 1996; 20: 35-43.
CrossRef - Bora K.S. and Sharma A. In vitro antioxidant and free radical scavenging potential of Medicago sativa Linn. J. Pharma. Res., 2010; 3: 1206-1210.\\
- Aurand L.W, Boone N.H. and Gidding G.G. Superoxide and singlet oxygen in milk lipid peroxidation. J. Dairy. Sci., 1977; 60: 363-369.
CrossRef - Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod., 2000; 63: 1035-1042.
CrossRef - Hochestein P. and Atallah A.S. The nature of oxidant and antioxidant systems in the inhibition of mutation and cancer. Res., 1988; 202: 363-375.
- Kappus H. Lipid peroxidation – Mechanism and biological relevance. In: Aruoma OI and Halliwell B. (eds.) Free radicals and food additives. Taylor and Francis, London, UK (1991) 59-75.
- Rahmat A.K, Mohammed R.H, Sumaira S. and Mushtaq. Evaluation of phenolic contents and antioxidant activity of various solvent extract of Sonchusasper ((L) Hill. Chemist. Cent. J., 2012; 6-12
- Siddhuraju P, Mohan P.S. and Becker K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruitpulp. Food. Chem., 2002; 79:61-67.
CrossRef - Yen G.C, Duh P.D. and Tsai C.L. Relationship between antioxidant activity and maturity of peanut hulls. J. Agr. Food. Chem., 1993; 41:67-70.
CrossRef - Chou H.J, Kuo J.T. and Lin E.S. Comparative antioxidant properties of water extracts from different parts of beefstreak plat (Perillafrutescens). J. Food. Drug. Anal., 2009; 17(6): 489-496.








