Manuscript accepted on :March 09, 2018
Published online on: --
Plagiarism Check: Yes
Laila A. Baqasi1,2, Huda A. Qari1,2 and Ibrahim A. Hassan2,3
1Department of Biology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
2Centre of Excellence in Environmental Studies (CEES), King Abdulaziz University, 21589 Jeddah, Saudi Arabia.
3Department of Botany, Faculty of Science, Alexandria University, 21589 El Shatby, Alexandria, Egypt.
Corresponding Author E-mail: ihassan_eg@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1346
Abstract
This study was to conducted to investigate the use of ethylenediurea (EDU) as a possible tool to evaluate O3 effects on wheat (Triticum aestivum L.) plants under field conditions in Jeddah. Wheat plants were expsoed to ambient O3 (AA) and the antiozonant chemical ethylenediurea (EDU) in closed fumigation chambers for the full growing season. Growth, yield and physiology were determined in response to O3 and/or EDU. EDU-treated plants had higher photosynthetic rates (24%) and stomatal conductance (25%), which were reflected in higher growth and yield in terms of number of grains. The present study revealed that EDU could be used as a promising tool to mitigate damaging effects of O3 on under field conditions. EDU protected wheat plants leading to increases in photosynthetic rates, growth and yield.
Keywords
Ethylenediurea (EDU); Growth; Ozone; Protection and Yield
Download this article as:| Copy the following to cite this article: Baqasi L. A, Qari H. A, Hassan I. A. Physiological and Biochemical Response of Winter Wheat (Triticum Aestivum L.) to Ambient O3 and the Antiozonant Chemical Ethylenediurea (EDU) in Jeddah, Saudi Arabia. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Baqasi L. A, Qari H. A, Hassan I. A. Physiological and Biochemical Response of Winter Wheat (Triticum Aestivum L.) to Ambient O3 and the Antiozonant Chemical Ethylenediurea (EDU) in Jeddah, Saudi Arabia. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19558 |
Introduction
Tropospheric O3 is produced through photochemical reactions of its precursors (NOx and VOCs) and its concentrations varied spatially and temporally. Ozone (O3) is a secondary phytotoxic pollutant causing changes in metabolic, biochemical and physiological processes leading to reductions in growth and yield of economic crops.1-4 Ozone concentrations in Jeddah were recorded to be between 40 – 70 nl l-1. 4,5 These concentrations are high enough to affect many plant processes, such as photosynthesis, transpiration, nutrient uptake, and senescence, resulting in significant effects on crop growth and yield.Jeddah suffers from serious air pollution problems due to emissions from different sources. There is a very poor legislation regarding emissions from old cars in streets as well from factories which cause environmental hazards. Levels of heavy metals, gaseous air pollutants and particulate emissions far exceed internationally acceptable standards.4-6 The antiozonant chemical ethylenediurea (EDU) is extensively used to assess the crop loss due to O3.7-8
Wheat is a nutritious and versatile crop, it has been known to be sensitive to O3 in the USA, Europe and other areas of the world.9-15 However, very little is known about its sensitivity in the Middle East.16 As yet, in Saudi Arabia, the information on impact of O3 on plants are extremely scanty and fragmented.5,17
The objective of the present investigation was to assess the response of local variety of wheat (Triticum aestivum L.) to ambient O3 in terms of growth, photosynthesis, stomatal conductance, antioxidants and photosynthetic pigments and to assess the use of the antiozonant chemical ethylenediurea abbreviated as EDU as a reliable, effective and cheap tool to protect of O3 effects on wheat plants under filed conditions in Jeddah, Saudi Arabia.
Materials and Methods
Plant Samples, Growth Conditions and Experimental Design
Grains of wheat (Triticum aestivum L.) were hand-planted in 50 cm2 plastic pots (10 seeds in each pot) filled with multipurpose in a controlled glasshouse. Plants were transferred to the field when second foliage leaf appeared on 3 Feb 2015 were sown. Experiments were conducted in the open field during Jan 2015 – April 2015. The treatments were: (a) ambient air (AA) and (b) AA + EDU (EDU). There were 12 pots/chamber. The crop matures in 70 – 80 days.
EDU Application
EDU was applied as a soil drench; 200 ml of a 150 ppm EDU every 14 days. The control plants received tap water. There were 16 plastic pots (18 cm height x 16 cm diameter); 8 for AA and 8 for EDU.
Biomass and Grain Yield
Five plants per pot were harvested for determination of yield parameters at the final harvest during April 2015.
Gas Exchange Measurements and Chlorophyll Content
Net CO2 photosynthetic rate (A) and stomatal conductance (gs) were measured using a portable LICOR (IRGA-LICOR-6400, Lincoln, NE, USA). Measurements were carried out on ten attached leaves per treatment on weekly basis.
Biochemical Assays and Antioxidant Enzymes Assays
Leaves were weighed and ground at about 4°C in 50 m Tris-HCl buffer containing 3 mM MgCl2, then the homogenates were centrifuged at 20 000 for 15 min (Centrifuge17 S/RS, Heraeus Sepatech). Enzyme assays were performed using the supernatants and the results were expressed on protein basis.
Four antioxidant enzymes were determined in the present investigation; namely; Superoxide dismutase (SOD), Catalase, Guaiacol peroxidase (GPX), Ascorbate (APX) following the methods as given by Lee et al.18
Data Analysis
ANOVA test was applied to data, differences between means were tested by Tuckey Test using SPSS statistical package, USA.
Results and Discussion
The average temperatures were 33.2°C and the average 8-h O3 concentrations were 45.9 ppb. Accumulated ozone exposure above a threshold of 40 ppb (AOT40 was calculated to be 3.304 ppm).
EDU-treated plant had higher photosynthesis rate (A) and stomatal conductance (gs) by 24%, increased by 25%, respectively, throughout the entire course of experiment (Fig. 1 A & B).
The rate of increase in photosynthetic rates due to application with EDU was steady for the first three weeks and increased thereafter and by the 8th week, it showed a slight decline due to senescence of the crop (Fig. 1A). On the other hand, the decline in stomatal conductance was more pronounced in the 8th week in both EDU-treated and ambient air plants (Fig. 1B).
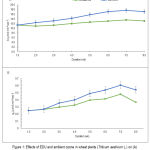 |
Figure 1: Effects of EDU and ambient ozone in wheat plants (Triticum aestivum L.) on (A) photosynthetic rate (A), and (B) stomatal conductance (gs). (n= 8 ± 1SE).
|
Plant treated with EDU showed a positive impact on number and weight of grains. EDU caused increases in the grain number by 50% and grain weight by 82% (Fig. 2 A & B).
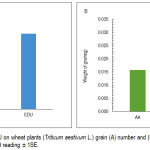 |
Figure 2: Effect of EDU on wheat plants (Triticum aestivum L.) grain (A) number and (B) weight. Each figure is a mean of 109 reading ± 1SE.
|
EDU did not alter shoot growth (P ˃ 0.05), while tit caused overall increase in root dry weight by 23% when compared to plants grown in ambient (AA) (Fig.3).
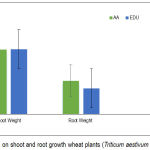 |
Figure 3: Effect of EDU on shoot and root growth wheat plants (Triticum aestivum L.). (Means ± 1SE).
|
Exposure to ambient O3 caused increases in Superoxide Dismutase (SOD), Ascorbate Peroxidase (APX) and Catalase (CAT) by 31, 16 and 27%, respectively (Table 1). Nevertheless, Guaiacol Peroxidase (GPX) showed insignificant increase 9.8% , (P˃ 0.05).
Table 1: Effects of EDU on superoxide dismutase, ascorbate peroxidase, guaiacol peroxidase and catalase in leaves wheat (Triticum aestivum L.) plants 70 days after planting.
| Enzyme | AA | EDU |
| SOD
(mg-1 protein) |
65.6 + 7.8b | 50.1 + 6.3a |
| APX
(µmol min-1 mg-1 protein) |
578.7 + 76.8a | 498.1 + 51.1b |
| GPX
(µmol min-1 mg-1 protein) |
8.02 + 1.01a | 7.3 + 1.9a |
| CAT
(µmol min-1 mg-1 protein) |
781.7 + 97.5a | 614.1 + 72.7b |
Each figure is a replicate of 8 reading ± 1SE. Mean not followed by the same letter are significantly different of P < 0.05.
Jeddah is the second largest city in the Kingdom of Saudi Arabia, suffering from industrial and vehicular emissions which cause increases in tropospheric O34,5.It is growing economically and industrially and having many anthropogenic activities; its population is about 3 million inhabitant.
The concentrations of O3 recorded in Jeddah is between 40 – 50 ppb were close to those previously observed in several arid areas of Saudi Arabia.4,5 Until now, one investigation on impact of ambient O3 and on crops was carried out in Saudi Arabia5 and the extent of economic losses of O3 to crops in this country has been poorly evaluated.
The present investigation showed clearly that O3 concentrations of Jeddah city cause negative effects on growth, biomass accumulation, and yield. Analyses of metabolic processes were performed in order to perceive O3 toxicity at early stages.
Ambient O3 significantly affected photosynthetic rates (A) values, in comparison to EDU-treated plants. This result is in agreement with our previous work1,3,5. Moreover, EDU-treated plants had higher stomatal conductance (gs) when compared to plants grown in ambient air.7-8
There is a significant correlation between A and gs (data not shown), which indicates that reduction in stomatal conductance due to closing of stomatal could be the cause of the apparent reduction of plants exposed to ambient air “AA”.5
EDU has been extensively used to assess the impact of ambient O3 in dry tropical/subtropical regions.2,5,19,20 However, there is only one study so far in the literature describing EDU application to crops in Saudi Arabia.5 EDU has no toxic effect per se.2,5 The positive effects of EDU were relevant on all variables of wheat plants in the present study, including O3– caused visible injury, growth, yield, and physiological parameters.
EDU caused increases in the biometric parameters (growth parameters, grain number and weight) compared to plants exposed to ambient conditions, approving its effectiveness in alleviating toxic effects of O3.
Plants can avoid and tolerate toxic effects of O3 through induction of protective scavenging systems.1,21 Plants produce antioxidant enzymes (e.g. catalase “CAT”, peroxidase “GPX”, superoxide dismutase “SOD” and others) and ascorbate peroxidase “APX”; the enzymes of the ascorbate-glutathione cycle (Halliwell-Asada cycle) in order to alleviate accumulation of harmful concentrations of reactive oxygen species (ROS) caused by O3.1
Oxidative stress causes increases in activities of these enzymes, which indicate production of H2O2. SOD plays a key protective role against O3 phytotoxicity, as it scavenges O2– and catalyzes its dismutation to H2O2 and O2.22,23 Both CAT and APX are scavengers of H2O2.24-26
Results of the present study point out that decreased activities of antioxidant enzymes in EDU-treated wheat plants most likely increased resistance of plants to O3. This protective mechanism of against harmful oxidative stress is reported in wheat plants in Saudi Arabia, in terms of levels of reactive oxygen species “ROS” and their analogous scavenging enzymes,.
In conclusion, EDU conferred a significant protection to wheat plants leading to improvement in growth, yield, and photosynthetic performance.
Acknowledgements
This work was supported with a Grant from King Abdul Aziz City for Science & Technology (KACST). Grant #1498-37-TA.
References
- Hassan I. A., Haiba N. S., Badr R. H., Basahi J. M., Almellebi T., Ismail I. M and Taia W. K. Effects of ambient ozone on reactive oxygen species and antioxidant metabolites in leaves of pea (Pisum sativum) Plants. Pak. J. Bot. 2017;49(1):47-55.
- Hassan I. A. Physiological and biochemical response of potato (Solanum tuberosum L. Cv. Kara) to O3 and antioxidant chemicals: possible roles of antioxidant enzymes. App. Bio. 2006;146:134– 142.
CrossRef - Ismail I. M., Basahi J. M., Hassan I. A. Gas exchange and chlorophyll fluorescence of pea (Pisum sativum) plants in response to ambient ozone at a rural site in Egypt. Sci. of the Tot. Environ. 2014;497–498:585–593.
CrossRef - Hassan I. A., Basahi J. M., Ismail I. M., Habeebullah T. M. Spatial distribution and temporal variation in ambient ozone and its associated NOx in the atmosphere of Jeddah City, Saudi Arabia. Aerosol and Air Qual. Res. 2013;13(6):1712–1722.
CrossRef - Basahi J. M., Ismail I. M., Haiba N. S., Hassan I. A., Lorenzini G. Assessing ambient ozone injury in olive (Olea europaea) plants by using the antioxidant ethylenediurea (EDU) in Saudi Arabia. Environ. Monit. Assess. 2016;188(6):371.
CrossRef - Hassan I. A., Basahi J. M. Assessing roadside conditions and vehicular missions using roadside lettuce plants. Polish J. Environ. Stud. 2013;22(2):75–81.
- Pasqualini S., Paoletti E., Cruciani G., Pellegrino R., Ederli L. Effects of different routes of application on ethylenediurea persistence in tobacco leaves. Pollut. 2016;212:559–564.
CrossRef - Pandey A. K., Majumder S., Keski-Saari S., Kontunen-Soppela A., Mishra N., Oksanen E. Searching for common responsive parameters for ozone tolerance in 18 rice cultivars in India: Results from ethylenediurea studies. Tot. Environ. 2015;532:230–328.
CrossRef - Feng Z., Kobayashi K., Ainsworth A. impact of elevated O3 concentration on growth, physiology and yield of wheat (Triticum aestivum): a meta-analysis. Global Change Bio. 2008;14:2696-2708.
- Feng Z., Pang J., Kobayashi K., Zhu J., Ort D. R. Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Global Change Bio. 2011;17(1):580–591.
CrossRef - Feng Z., Wang L., Pleijel H., Zhu J., Kobayashi K. Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Tot. Environ. 2016;572:404–411.
CrossRef - Mishra K., Rai R., Agrawal S. B. Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian J. Biochem. Biophys. 2013;50(2):139–49.
- Liu X., Sui L., Huang Y., Geng C., Yin B. Physiological and visible injury responses in different growth stages of winter wheat to ozone stress and the protection of spermidine. Pollut. Res. 2015;6(4):596–604.
CrossRef - Burkart S., Bender J., Tarkotta B., Faust S., Castagna A., Ranieri A., Weigel H. J. Effects of Ozone on Leaf Senescence, Photochemical Efficiency and Grain Yield in Two Winter Wheat Cultivars. of Agro. Crop Sci. 2013;199(4):275–285.
CrossRef - Yi F., Jiang F., Zhong F., Zhou X., Ding A. The impacts of surface ozone pollution on winter wheat productivity in China – An econometric approach. Environmental Pollution. 2016;208:326–35.
CrossRef - Hatata M., Badr R., Ibrahim M., Hassan I. A. Respective and interactive effects of O3, CO2 an drought stress on physiology and antioxidative ability and yield of wheat plants. Current World Env. 2013;8(3):421-429.
CrossRef - Al-Qurainy F. H. Effect of air pollution and Ethylenediurea on broad bean plants grown at two localities in KSA. International Journal of Bot. 2008;4(1):117–122.
CrossRef - Lee E. H., Upadhaya A., Agrawal M., Rowland R. A. Mechanism of EDU-induced O3 protection: re-examination of free radical scavenger system in snap bean exposed to O3. Expert. Botany. 1997;38:199-209.
- Manning W. J., Paoletti E., Sandermann H., Ernst D. Ethylenediurea (EDU) a research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Pollut. 2011;159:3283–3293.
CrossRef - Agrawal S. B., Singh A., Rathore D. Role of EDU in assessing impact of O3 on Vinga radiata plants in suburban area of Allahabad (India). Chemosphere. 2005;61:218–228.
CrossRef - Zengin F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. Environ. Bio. 2015;36:249-254.
- Sarkar A., Agrawal S. B. Elevated ozone and two modern wheat cultivars: an assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Exper. Bot. 2010;69;328–337.
CrossRef - Singh A. A., Singh S., Agrawal M., Agrawal S. B. Assessment of ethylene diurea-induced protection in plants against ozone phytotoxicity. Reviews Environ. Contam.Toxi. 2014;233;129–184.
CrossRef - Cho K., Tiwari S., Agrawal S. B., Torres N. L., Agrawal A., Sarkar J., et al. Tropospheric ozone and plants: absorption, responses, and consequences. Environ. Contam. Toxicol.; 212: 111–116 (2011).
CrossRef - Wang J., Zeng Q., Zhu J., Chen C., Liu G., Tang H. Apoplastic antioxidant enzyme responses to chronic free-air ozone exposure in two different ozone-sensitive wheat cultivars. Pollut. 2014;148(2):390–39.
CrossRef - Pellegrini E., Francini A., Lorenzini G., Nali C. Ecophysiological and antioxidant traits of Salvia officinalis under ozone stress. Environ. Sci. Pollut. Res. 2015;22:13083–13093.
CrossRef







