Omar M. E. Abdel-Salam1 , Amany A. Sleem4
, Amany A. Sleem4 , Eman R. Youness2
, Eman R. Youness2 , Nadia A. Mohammed2
, Nadia A. Mohammed2 and Enayat A. Omara3
and Enayat A. Omara3
1Department of Toxicology and Narcotics, National Research Centre, Cairo, Egypt.
2Department of Medical Biochemistry, National Research Centre, Cairo, Egypt.
3Department of Pathology, National Research Centre, Cairo, Egypt.
4Department of Pharmacology, National Research Centre, Cairo, Egypt.
Corresponding Author E-mail: omasalam@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1343
Abstract
We studied the effect of bone marrow-derived stem cells (BM-SCs) on oxidative stress, inflammation and pathological changes induced in the brain and liver of mice by the antipsychotic drug haloperidol. Mice were intraperitoneally (i.p.) treated with haloperidol at 5 mg/kg for 3 consecutive days followed by i.p. stem cell suspension and euthanized 24h later. Haloperidol resulted in increased brain and liver malondialdehyde (MDA) and nitric oxide contents together with decreased reduced glutathione (GSH). There were also decreased paraoxonase-1 (PON-1) activity in brain and liver and increased interleukin-1β (IL-1 β), interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α) in brain tissue. Haloperidol produced neuronal necrosis and apoptosis and the appearance of esinophilic areas and strong TNF-α immunoreactivity in the cerebral cortex and striatum of treated mice. In the liver, centrilobular necrosis, inflammatory cell infiltration and sinusoidal haemorrhage were observed. In haloperidol-treated mice, stem cell injection had no significant effects on brain and liver levels of MDA, nitric oxide or GSH. Paraoxonase-1 activity in brain, however, decreased by stem cells application. In brain, there were decreased IL-1β, IL-6 and TNF-α. Brain neurodegenerative changes, brain TNF-immunoreactivity and histological liver damage were all markedly ameliorated after stem cell treatment. These results indicate that stem cells protect against brain and liver toxicity caused by short term haloperidol treatment in high dose. The protective effects of stem cell treatment is likely to result from interfering with cytokine release.
Keywords
Haloperidol; Interleukin;Oxidative Stress; Paraoxonase-1; Stem Cells; Tumour Necrosis Factor-Alpha
Download this article as:| Copy the following to cite this article: Abdel-Salam O. M. E, Sleem A. A, Youness E. R, Mohammed N. A, Omara E. A. Bone Marrow-Derived Protect Against Haloperidol-Induced Brain and Liver Damage in Mice. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Abdel-Salam O. M. E, Sleem A. A, Youness E. R, Mohammed N. A, Omara E. A. Bone Marrow-Derived Protect Against Haloperidol-Induced Brain and Liver Damage in Mice. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19546 |
Introduction
Haloperidol is a classic antipsychotic drug in use to treat symptoms of schizophrenia. The drug acts by inhibiting dopamine D2 receptors (D2, 3, 4) in mesolimbic dopaminergic pathway1 and is effective in improving the positive symptoms i.e., delusions and hallucinations.2 Haloperidol also binds to striatal D2 receptors with high occupancy and with an increased propensity of inducing extrapyramdial manifestations eg., tardive dyskinesia.3 The drug in addition is likely to impair cognition compared to the newer generation antipsychotic agents.4 Haloperidol causes brain oxidative stress,5-7 a situation where the increased generation of reactive oxygen metabolites overwhelms the cell’s antioxidant mechanisms with a resultant oxidative damage to cell membrane lipids, enzymes, and nucleic acids.8 When given to rats, haloperidol resulted in increased lipid peroxidation, depletion of reduced glutathione, increased nitric oxide in several brain regions7,9,10 and caused neuronal damage.11,12 Haloperidol thus despite its ability to improve symptoms of schizophrenia, might in the same time increase the susceptibility of the brain tissue to neurodegeneration via causing oxidative stress. The latter has been linked to the development of schizophrenic symptoms13,14 and there is evidence suggesting a benefit from administering antioxidant molecules to schizophrenic subjects.15-17 The presence of increased concentrations of the pro-inflammatory cytokines INF-g and NF-α in the brain of schizophrenic subjects also indicates the presence of an ongoing inflammatory process18-20 and this could be exacerbated by haloperidol.
Stem cells are characterized by self-renewal and the ability to differentiate into a variety of cell lineages.21 They are classified into embryonic stem cells, embryonic germ cells and adult stem cells that can be found in bone marrow, blood, adipose tissue, brain, and liver.21,22 Research on stem cells has attracted much interest for their possible application in the treatment of many neurological diseases. Replacing diseased or damaged brain cells with other healthy cells is in itself an old hope and requirement.23 Recently, however, mounting evidence suggest that the benefit from stem cells might derive from an anti-inflammatory and immune-modulating actions.24 Mesenchymal stem cells (MSCs) can be isolated from adult tissues such as bone marrow, adipose tissue, muscle, liver, and skin.21 When given intravenously, these cells have demonstrated a therapeutic potential in experimental models of traumatic brain injury,24,25 Parkinson’s disease,26 fulminant hepatic failure,27 liver fibrosis,28 graft-versus-host disease29 and ischaemic acute renal failure.30 In these studies, stem cell therapy have shown to exert acute anti-inflammatory, antiapoptotic and immune modulating effects that are largely thought to account for the therapeutic benefit.
In view of the above, the present study aimed to investigate the possible effects of stem cell therapy on the oxidative stress and inflammatory status after acute haloperidol administration in mice with a view to possibly reducing the side effects of the classic antipsychotic.
Materials and Methods
Animals
Studies were conducted using Swiss male albino mice 22–25 g of body weight and aged 5– 6 weeks. Mice were obtained from the animal house colony of the National Research Centre (Cairo, Egypt), housed on a 12-h light/dark cycle and allowed free access to standard laboratory food and tap water. Animal procedures were done according to the Ethics Committee of the National Research Centre and the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Drugs and Chemicals
Haloperidol was obtained from Kahira Pharm and Chem. IND (Cairo, Egypt) and dissolved in sterile physiological saline. Stem cell isolates were obtained from Cairo University. All other chemicals were of analytical grade and purchased from Sigma.
Study Design
Mice were randomly divided into 4 equal groups (6 mice each). The first 3 groups were treated with haloperidol (5 mg/kg, i.p., 0.1 ml) for 3 consecutive days and then received either physiological saline (0.2 ml) or stem cell suspension (2 × 105 BM-SCs, 0.2 ml). The 4th group was treated with only saline (i.e. no haloperidol). Mice were euthanized 4h after stem cell injection by decapitation under ether anesthesia. The brain and liver of each mouse were then quickly removed on ice plates, washed with ice-cold phosphate buffered saline (PBS, pH 7.4), weighed, and then stored at−80°C. The brain and liver tissues were homogenized with 0.1 M phosphate buffer saline at pH 7.4 to give a final concentration of 10 %w/v for the biochemical assays.
Stem Cell Isolation
Bone marrow was harvested by flushing the rat tibiae and femurs with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Nucleated cells were then isolated with a density gradient and re-suspended in a complete culture medium supplemented with 1% penicillin–streptomycin. Cells were incubated at 37°C in 5% humidified CO2 for a period of 12–14 days as a primary culture. Following the development of large colonies, cultures were then washed twice with phosphate buffer saline and the cells trypsinized with 0.25% trypsin in 1mM EDTA for 5 min at 37°C. After centrifugation cells were re-suspended in serum supplemented medium and incubated in 50 cm2 culture flask. The resulting cultures were referred to as first-passage cultures. The identification of cells as being MSCs was based on morphology, adherence and the ability to differentiate into osteocytes and chondrocytes.31
Determination of Lipid Peroxidation
Lipid peroxidation was assayed by measuring the level of malondialdehyde in supernatants using the method of Ruiz-Larrea et al.32 In this assay thiobarbituric acid reactive substances (TBARS) react with thiobarbituric acid to produce a red-colored complex having peak absorbance at 532 nm.
Determination of Reduced Glutathione
Reduced glutathione was measured in tissue supernatants using the method of Ellman.33 In this assay, Ellman´s reagent is reduced by –SH groups of GSH to form 2-nitro-s-mercaptobenzoic acid. The nitromercaptobenzoic acid anion with an intense yellow color can then be determined spectrophotometrically.
Nitrite Determination
The measurement of nitric oxide in supernatants was done according to the method of Moshage et al.34. In this assay nitrite is used as indicator for the production of nitric oxide.
Determination of Paraoxonase Activity
Arylesterase activity of paraoxonase was measured spectrophotometrically in supernatants using phenylacetate as a substrate. Arylesterase/paraoxonase catalyzes the cleavage of phenyl acetate resulting in phenol formation. The rate of formation of phenol is measured by monitoring the increase in absorbance at 270 nm at 25°C.35,36
Quantification of IL-1β
Interleukin-1beta (IL-1β) was measured in tissue supernatants using commercially available IL-1 beta ELISA kit (KOMA BIOTECH Inc.) according to manufacture instructions. The kit uses a double antibody sandwich enzyme linked immunosorbent assay to measure the level of IL-1β.
Quantification of IL-6
Interleukin-6 (IL-6) was assayed in tissue supernatants using a double-antibody sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Quantification of TNF-α
The level of TNF-α in tissue supernatants was determined with a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit supplied by Thermofischer Scientific Co, USA according to the manufacture instructions.
Histopathology
Brain and liver sections were fixed in freshly prepared 10 % neutral buffered formalin, processed routinely, and embedded in paraffin. Paraffin sections (5 mm thick) were prepared and stained with haematoxylin and eosin (H&E) for the histopathological examination. Sections were examined using a light microscope (Nikon, Japan).
Immunohistochemistry
For immunohistochemistry 4 μm thick deparaffinized tissue sections were used. Briefly, deparaffinized slices were incubated overnight with antibodies against inducible TNF-α (diluted 1:100). Endogenous peroxidase activity was blocked by incubation in 0.075% hydrogen peroxide in PBS. For antibody detection DAKO EnVision+ System, Peroxidase/DAB kit was used. The sections were counterstained with haematoxylin, dehydrated with graded alcohols and xylene, and mounted on slides. The immunostaining intensity and cellular localization TNF-α was studied using light microscopy.
Statistical Analysis
Data are presented as mean ± SE. The data are analyzed by one way analysis of variance, followed by Duncan’s multiple range test for post hoc comparison of group means. Effects with a probability of p<0.05 are considered significant.
Results
Biochemical Results
Oxidative Stress Markers
Mice treated with haloperidol alone had significantly higher brain malondialdehyde and nitric acid contents by 35.1% and 48.2% compared with the saline control group. There was a 28.7% and 36.1% decrease in reduced glutathione PON-1 activity, respectively. In addition, haloperidol treatment caused significant increase in the liver malondialdehyde and nitric oxide levels by 60.5% and 58.4%, respectively. Significantly decreased reduced glutathione by 36% and PON-1 activity by 32.4% were also observed in the liver of haloperidol injected mice compared with the saline group. The administration of BM-SCs had no significant effect on the concentration of malondiadehyde, nitric oxide, reduced glutathione in the brain and liver tissue of haloperidol treated mice. BM-SCs administered after haloperidol treatment, however, decreased brain PON-1 activity by 18.2% compared with haloperidol only treatment (Table 1).
Table 1: Effect of single injection of bone marrow derived stem cells (BM-SCs) on oxidative stress in brain of mice treated with haloperidol (5 mg/kg) for 3 consecutive days.
| Saline | Haloperidol | Haloperidol + BM-SCs | |
| MDA (nmol/g. tissue) | 25.1 ± 0.96 | 33.9 ± 1.25* | 32.7 ± 2.00* |
| Nitric oxide (mmol/g. tissue) | 22.0 ± 1.43 | 32.6 ± 2.4* | 32.9 ± 2.1* |
| GSH (mmol/g. tissue) | 4.08 ± 0.34 | 2.91 ± 0.11* | 3.11 ± 0.19* |
| PON1 (kU/l) | 14.35 ± 0.93 | 9.17 ± 0.51* | 7.50 ± 0.82*+ |
Results are presented as mean ± SEM. *p<0.05 vs. saline control group
Table 2: Effect of single injection of bone marrow derived stem cells (BM-SCs) on oxidative stress in liver of mice treated with haloperidol (5 mg/kg) for 3 consecutive days.
| Saline | Haloperidol | Haloperidol + BM-SCs | |
| MDA (nmol/g. tissue) | 39.5 ± 1.5 | 68.38 ± 3.9* | 64.8 ± 2.3* |
| Nitric oxide (mmol/g. tissue) | 43.8 ± 3.0 | 69.4 ± 5.6* | 71.6 ± 3.2* |
| GSH (mmol/g. tissue) | 9.46 ± 0.68 | 6.06 ± 0.41* | 5.78 ± 0.65* |
| PON1 (kU/l) | 39.35 ± 2.23 | 26.60 ± 1.52* | 30.1 ± 1.90* |
Results are presented as mean ± SEM. *p<0.05 vs. saline control group
Inflammatory markers
The concentrations of IL-1β, IL-6, and TNF-α were markedly and significantly elevated in the brain of haloperidol treated mice by 5.11.6%, 344.3% and 249.7%, respectively, compared with the saline group. The administration of BM-SCs significantly reduced IL-1β, IL-6, and TNF-α concentrations by 21.9%, 16.9%, 30.2%, respectively, compared with the haloperidol treated group (Table 2).
Table 3: Effect of single injection of bone marrow derived stem cells (BM-SCs) on brain IL-1β, IL-6 and TNF-α in mice treated with haloperidol (5 mg/kg) for 3 consecutive days.
| Saline | Haloperidol | Haloperidol + BM-SCs | |
| IL-1β (Pg/ml) | 61.00 ± 2.70 | 373.11 ± 8.92* | 291.52 ± 7.43*+ |
| IL-6 (Pg/ml) | 4.40 ± 0.23 | 19.55 ± 0.81* | 16.25 ± 0.53*+ |
| TNF-α (Pg/ml) | 18.30 ± 0.76 | 64.00 ± 3.80* | 44.70 ± 0.25*+ |
Results are presented as mean ± SEM. *p<0.05 vs. saline control group. +*p<0.05 vs. haloperidol control group.
Histopathological Results
Brain Tissue
In the cortex and striatum of the saline-treated control group the normal structure and neuronal cells with prominent nuclei were observed (Fig.1 & 2 A). Haloperidol caused disorganization of cortical layers, degeneration, shrinkage of neurons, neuronal necrosis, vacuolation of neuropil, eosinophilic areas and pyknosis with apoptotic cells in the cortex and striatum. Perivascular space was found to be increased or dilated along with focal cerebral hemorrhage inside the brain parenchyma (Fig. 1, 2 B & C). However, light microscopic examination of these brain regions after treatment with stem cell suspension, showed return of brain tissues towards normal morphology as evidenced by remarkable regression of the total degenerative changes induced by haloperidol, although pyknosis of some neurons was still seen (Fig. 1, 2 D).
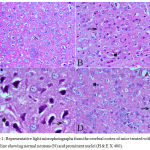 |
Figure 1: Representative light microphotographs from the cerebral cortex of mice treated with; (A) Saline showing normal neurons (N) and prominent nuclei (H & E X 400).
|
(B) Haloperidol: cytoplasmic vacuoletion (V), degeneration of neural cells (arrow), and pyknotic darkly stained nuclei (P) with apoptotic cells (arrowhead)(H & E X 400). (C) Haloperidol group: cytoplasmic vacuoletion (V), degeneration of neural cells (arrow), and pyknotic darkly stained nuclei (P) with apoptotic cells (arrowhead).(H & E X 1000). (D) Haloperidol and stem cells: few degenerated neurons (arrow), hemorrhage (H) with few pyknotic nuclei (P) (H & E X 400)
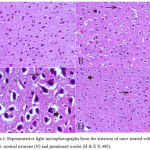 |
Figure 2: Representative light microphotographs from the striatum of mice treated with; (A) Saline: normal neurons (N) and prominent nuclei (H & E X 400).
|
(B) Haloperidol: vacuolation of neuropil (V), red neurons (R), pyknotic darkly (arrow) stained and apoptotic nuclei (arrowhead). (C) Haloperidol: shrunken with vacuolation of neuropil (V) and red neurons (R). Pyknotic darkly (arrow) stained and apoptosis nuclei (arrowhead) (H & E X 1000). (D) Haloperidol and stem cells: few pyknotic (arrow) and apoptosis nuclei (arrowhead) (H & E X 400).
Liver Tissue
Sections from saline treated mice showed normal hepatic architecture with distinct hepatic cells, central vein, sinusoidal spaces and prominent nuclei (Fig.3A). Haloperidol resulted in distortion in hepatocyte arrangement, obvious centrilobular necrosis with degeneration, vacuolation with the nuclei appearing pyknotic in these cells. Inflammatory cell infiltration especially in the periportal area, dark eosinophilic cytoplasm, dilation and sinusoidal hemorrhage with activation of Kupffer cell were also observed (Fig. 3B & C). In the group treated with haloperidol and stem cell suspension, there was almost normalization of the liver tissue and normalized cellular arrangement around the central vein, reduced necrosis with mild dilatation of sinusoids and no congestion. The nuclei of the liver were nearly normal indicating the recovery of the liver tissues, except few pyknotic cells (Fig. 3 D).
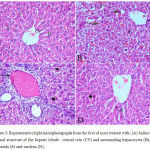 |
Figure 3: Representative light microphotographs from the liver of mice treated with; (A) Saline: normal structure of the hepatic lobule: central vein (CV) and surrounding hepatocytes (H), sinusoids (S) and nucleus (N).
|
(B) Haloperidol: distortion of hepatic architecture, necrosis with degeneration of hepatocytes with pyknotic nuclei. Dilation and hemorrhage of sinusoids. (C) Haloperidol group: distortion of hepatic architecture, inflammatory cell infiltration especially in the periportal area, dark eosinophilic cytoplasm, degeneration of hepatocytes with pyknotic nuclei. (D) Haloperidol and stem cells: normal hepatic cells with dilatation of hepatic sinusoids (S) and pyknotic nuclei (P) (H & E X 400)
Tumour Necrosis Factor Immunoreactivity
The cerebral cortex and striatum of the saline control group showed negligible staining for TNF-α expression (Figs. 4 D & G). Strong TNF-α immunoreactivity in the cytoplasm and nuclei of neurons was, however, seen in mice treated with haloperidol (Figs. 4 E & H). TNF-α expression decreased to nearly normal levels in the haloperidol and stem cells-treated group as compared to that of the haloperidol only group. Only a few scattered TNF-α-positive cells were observed (Fig. 4 F & I).
In the liver tissue TNF-α immunoreactivity was not detected in saline control group (Fig. 4 A). In the haloperidol group, strong TNF-α expression was seen mainly in the necrotic areas and predominantly in pericentral and periportal areas (Fig. 4B). Mice treated with haloperidol and stem cells showed markedly reduced TNF-α immunoreactivity (Fig. 4C).
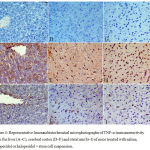 |
Figure 4: Representative Immunohistochemical microphotographs of TNF-α immunoreactivity from the liver (A–C), cerebral cortex (D–F) and striatum (G–I) of mice treated with saline, haloperidol or haloperidol + stem cell suspension.
|
A–C: liver tissue: (A) Saline-treated mice: there was no TNF-α expression. (B) Haloperidol treatment: strong TNF-α immunoreactivity. (C) Haloperidol + stem cells: markedly reduced TNF-α immunoreactivity.
D–F: cerebral cortex: (A) Saline-treated mice: no TNF-α immunoreactivity. (B) Haloperidol treatment: TNF-α immunopositivity was strongly increased. (C) Haloperidol + stem cells: few TNF-α-immunoreactive neurons.
G–I: striatum: (A) Saline-treated mice: no TNF-α immunoreactivity. (B) Haloperidol treatment: strong TNF-α immunoreactivity. (C) Haloperidol + stem cells: few TNF-α-immunoreactive neurons. (TNF-α, hematoxylin counterstain ×400 and brown color indicates TNF-positivity).
Discussion
In this study, haloperidol given at 5 mg/kg for three consecutive days produced neuronal necrosis and apoptosis in the cerebral cortex and striatum as well as hepatic centrilobular necrosis with inflammatory cell infiltration and sinusoidal haemorrhage. We also found that single systemic administration of BM-MSCs suspension was able to ameliorate brain and liver tissue damage caused by acute haloperidol injection in mice. The study thus provides further evidence for a neuro- and hepatic protective effect for MSCs. The administration of haloperidol in the dose of 5 mg/kg was found to cause lipid peroxidation indicated by the rise in tissue malondialdehyde level. There was also depletion of the intracellular antioxidant and the free radical scavenger reduced glutathione which is likely to reflect its consumption by the increased generation of free radicals. The results obtained are in agreement with other studies indicating increased brain and liver oxidative stress after haloperidol administration in rodents.5,9,7,10,37 In these studies, chronic haloperidol administration caused increased malondialdehyde and depletion of reduced glutathione in several brain regions and in the liver.7,9 The drug also increased superoxide in striatum37 and decreased brain superoxide dismutase activity,12,38 glutathione peroxidase activity12, catalase activity and total antioxidant capacity.10 In vitro, haloperidol increased the levels of TBARS when incubated with plasma from healthy volunteers.39 In this study, we also found increased brain and liver tissue nitric oxide by haloperidol. These observations confirm other studies that showed increased nitric oxide increased in whole brain and cortex after chronic treatment with haloperidol in mice and rats.7,10 The increased tissue nitric oxide by haloperidol could be involved in the observed tissue damage via the formation of more reactive oxygen and nitrogen species by reacting with molecular oxygen. When present in high amounts, nitric oxide can react with the superoxide anion (O2·-) resulting in the highly reactive peroxynitrite (ONOO−) capable of oxidation of thiols or protein thiols or nitration of protein tyrosine residues. Other reactive nitrogen oxides such as nitrogen dioxide (•NO2) and dinitrogen trioxide (N2O3) can nitrosate thiols or protein cysteine residues.40
The results from this study also support previous work indicating significantly decreased paraoxonase-1 (PON-1) activity in brain and liver after haloperidol treatment in rats.10 The enzyme PON-1 which is synthesized by the liver is involved in the detoxification of some organophosphate insecticides and nerve agents.41 Moreover, PON-1 has been found to have an antioxidant and anti-inflammatory roles36,42 and decreased activity was reported in such conditions as such as dementia,43 Alzheimer’s disease44 and autism.45 Plasma serum arylesterase activity also fell in first episode schizophrenic patients.46,47 Paraoxonase-1 is sensitive to oxidative stress48 and decreased activity might thus reflect inactivation by reactive oxygen metabolites.
The present work show that the acute haloperidol treatment results in neuronal necrosis and apoptosis and eosinophilic areas in the cerebral cortex and striatum of mice. Increased number of apoptotic cells in the striatum has been reported following administration of a single dose of 4mg/kg haloperidol in rats. The higher dose of 12 mg/kg resulted in increased number of apoptotic cells in both the striatum and substantia nigra pars reticulata.11 In vitro treating HT-22 cells with haloperidol resulted in increased number of apoptotic cells (TUNEL staining) and increased the expression of pro-apoptotic (Bax) protein in the hippocampus and caudate putamen of treated rats.49 Neuronal atrophy and necrosis in the cerebral cortex and sunstantia nigra has also been reported following chronic haloperidol treatment at a dose of 2 mg/kg for 21 days.12 Haloperidol thus causes neuronal damage which could be due to increased oxidative/nitrosative burden. The latter process can result in consequent impairment of mitochondrial respiration and brain energy depletion.50,51 Our findings are important in view of the data implicating increased oxidative stress in schizophrenia.13,14 In these patients there is increased malondialdehyde46 and F2-isoprostane levels52 in plasma as well as increased urinary excretion of RNA and DNA oxidation products.53 Studies have also shown significantly higher malondialdehyde levels in the plasma of schizophrenia patients treated with first generation antipsychotics like haloperidol compared to second generation agents.54
There is ample evidence that suggests a role for pro-inflammatory cytokines in the pathogenesis of schizophrenia. Thus, first episode schizophrenia showed increased serum or plasma IL-1β,55 IL-6, TNF-α,18,19,47 IL-447 and IL-1856 in first episode schizophrenia patients compared with healthy controls. Schizophrenia patients with acute exacerbation of exhibited increased plasma IL-6 levels compared with patients in remissions.57 In addition, increased IL-6 mRNA in mononuclear cells from peripheral blood has also been demonstrated in schizophrenics with worse positive symptoms.20 In this study, the concentrations of IL-1β, IL-6 and TNF-α were measured in brain tissue of mice after treatment with haloperidol. We found significantly increased concentrations of the studied pro-inflammatory cytokines compared to the control group. We also demonstrated strong TNF-α immunoreactivity in the cerebral cortex and striatum of haloperidol treated mice compared to controls. Haloperidol might thus be able to exacerbate the inflammatory process in the schizophrenic brain and consequently the progression to neurodegeneration.
In this study, the systemic administration of BM-MSCs caused remarkable regression of the neurotoxic and hepatotoxic effects of haloperidol as evidenced by the histologic evaluation of brain and liver tissue after BM-MSCs treatment. In both the brain and liver tissue of haloperidol-treated mice, lipid peroxidation, nitric oxide or reduced glutathione were not altered by BM-MSCs treatment. Brain PON-1 activity, however, showed further decrease after stem cell therapy. Thus, the protective effect of BM-MSCs was not due to an antioxidant action. Stem cell therapy, however, exerted anti-inflammatory action causing significant decrease in the levels of the proinflammatory cytokines IL-1β, IL-6 and TNF-α in brain of haloperidol treated mice. Our results are therefore in favor for an anti-inflammatory action for stem cell suspension in decreasing haloperidol-induced neuronal and liver damage. In previous studies, i.p. MSCs of bone marrow or adipose tissue origin were shown to ameliorate neuronal damage in different brain regions and also hepatocyte degeneration and liver inflammation caused by lipopolysaccharide (LPS) endotoxin in mice. The oxidative stress response was not reduced but there were decreased expression of caspase-3, cyclooxygenase-2, TNF-α and inducible nitric oxide synthase in brain and liver tissue by MSCs treatment.58,59 MSCs injected i.v. were able to ameliorate the behavioral changes and the loss in tyrosine hydroxylase positive neurons in the substantia nigra pars compacta in an experimental model of Parkinson’s disease via an anti-apoptotic action.26 In experimental traumatic brain injury, i.v. MSCs reduced the release of cytokines into the brain tissue.25 In a model of fulminant hepatic failure, i.v. MSCs reduced mortality, hepatocyte dealth and tissue leucocytic infiltration.27 MSCs injected i.v. were also shown to modulate the inflammatory response in renal injury reducing the expression levels of IL-6, TNF-α mRNA and decreasing serum IL-1α, IL-1β, INF-g and INF-α. 60 It is thus becoming clear that MSCs exert their therapeutic effects via an anti-apoptotic action and by modulating the inflammatory response.
In summary, the present study demonstrate that BM-MSCs given i,p. can protect against brain injury and hepatocyte death caused by short term haloperidol treatment in high dose in mice. This therapeutic benefit of BM-MSC is likely to be mediated by interfering with the inflammatory response.
Acknowledgements
This works is was not supported by research grants
Conflicts of Interest
There is no conflict of interest.
References
- Miller B. J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671.
CrossRef - Labarca R., Silva H., Jerez S., Ruiz A., Forray M. I., Gysling K., Andres M. E., Bustos G., Castillo Y., Hono J. Differential effects of haloperidol on negative symptoms in drug-naive schizophrenic patients: effects on plasma homovanillic acid. Schizophr Res. 1993;9(1):29-34.
CrossRef - Miyamoto S., Duncan G. E., Marx C. E., Lieberman J. A. Treatments for schizophrenia a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry. 2005;10:79–104.
CrossRef - Meltzer H. Y., Park S., Kessler R. Cognition, schizophrenia, and the atypical antipsychotic drugs. Proc Natl Acad Sci U S A. 1999;96(24):13591–13593.
CrossRef - Post A., Holsboer F., Behl C. Induction of NF-kB activity during haloperidol-induced oxidative toxicity in clonal hippocampal cells: suppression of NF-kB and neuroprotection by antioxidants. J Neurosci. 1998;18(20):8236–8246.
CrossRef - Martins M. R., Petronilho F. C., Gomes K. M., Dal-Pizzol F., Streck E. L., Quevedo J. Antipsychotic-induced oxidative stress in rat brain. Neurotox Res. 2008;13:63-9.
CrossRef - Abdel-Salam O. M., El-Sayed El-Shamarka M., Salem N. A., El-Mosallamy A. E., Sleem A. A. Amelioration of the haloperidol-induced memory impairment and brain oxidative stress by cinnarizine. EXCLI J. 2012;11:517-30.
- Halliwell B., Gutteridge J. M. C. Free radicals in biology and medicine, 3rd edn. Clarendon Press, Oxford. 199.
- Vairetti M., Feletti F., Battaglia A., Pamparana F., Canonico P. L., Richelmi P., Bertè F. Haloperidol-induced changes in glutathione and energy metabolism: effect of nicergoline. Eur J Pharmacol. 1999;367:67-72.
CrossRef - Abdel-Salam O. M., Youness E. R., Khadrawy Y. A., Sleem A. A. Brain and liver oxidative stress after sertraline and haloperidol treatment in mice. J Basic Clin Physiol Pharmacol. 2013;24(2):115-23.
CrossRef - Mitchell I. J., Cooper A. C., Griffiths M. R., Cooper A. J. Acute administration of haloperidol induces apoptosis of neurones in the striatum and substantia nigra in the rat. Neuroscience. 2002; 109(1):89-99.
CrossRef - Perera J., Tan J. H., Jeevathayaparan S., Chakravarthi S., Haleagrahara N. Neuroprotective effects of alpha lipoic acid on haloperidol-induced oxidative stress in the rat brain. Cell Biosci. 2011;1(1):12.
CrossRef - Wu J. Q., Kosten T. R., Zhang X. Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry .2013;46:200-206.
CrossRef - Flatow J., Buckley P., Miller B. J. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409.
CrossRef - Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Judd F., Katz F., Katz P., Ording-Jespersen S., Little J., Conus P., Cuenod M., KQ D., Bush A. I. N-acetyl cysteine as a glutathione precursor for schizophrenia-a double-blind, randomized placebo-controlled trial.Biol Psychiatry. 2008;64(5):361-8.
CrossRef - Dean O., Giorlando F., Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36(2):78-86.
CrossRef - Bošković M., Vovk T., Koprivšek J., Plesničar B. K., Grabnar I. Vitamin E and essential polyunsaturated fatty acids supplementation in schizophrenia patients treated with haloperidol. Nutr Neurosci. 2016;19(4):156-61.
CrossRef - O’Brien S. M., Scully P., Dinan T. G. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160(3):256-62.
CrossRef - Kubistova A., Horacek J., Novak T. Increased interleukin-6 and tumor necrosis factor alpha in first episode schizophrenia patients versus healthy controls. Psychiatria Danubina. 2012;24(1):153–156.
- Chase K. A., Cone J. J., Rosen C., Sharma R. P. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
CrossRef - Weiner L. P. Definitions and criteria for stem cells. Methods Mol Biol. 2008;438:3-8.
CrossRef - Verfaillie C. M. Multipotent adult progenitor cells: an update. Novartis Found Symp. 2005;265:55-61.
CrossRef - Ramsay M. A. E. Will stem cells transform medicine? Proc (Bayl Univ Med Cent). 2002;15:135–137
CrossRef - Zhang R., Liu Y., Yan K., Chen L., Chen X. R., Li P., Chen F. F., Jiang X. D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106.
CrossRef - Galindo L. T., Filippo T. R. M.,Semedo P., Ariza C. B., Moreira C. M., Camara N. O. S., Porcionatto M. A. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. 2011:564089.
CrossRef - Wang F., Yasuhara T., Shingo T., Kameda M., Tajiri N., Yuan W. J., Kondo A., Kadota T., Baba T., Tayra J. T., Kikuchi Y., Miyoshi Y., Date I. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci 2010;11:52.
CrossRef - Parekkadan B., van Poll D., Suganuma K., Carter E. A., Berthiaume F., Tilles A. W., Yarmush M. L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9):e941.
CrossRef - Zhao W., Li J. J., Cao D. Y., Li X., Zhang L. Y., He Y., Yue S. Q., Wang D. S., Dou K. F. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18(10):1048-1058.
CrossRef - Yañez R., Lamana M. L., García-Castro J., Colmenero I., Ramírez M., Bueren J .A. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24(11):2582-91.
CrossRef - Cao H., Qian H., Xu W., Zhu W., Zhang X., Chen Y., Wang M., Yan Y., Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32(5):725-32.
CrossRef - Aziz M. T. A., Wassef M. A., Rashed L. A., Mhfouz S., Omar N., Elsebaie M. M. Mesenchymal stem cells therapy in acute renal failure: possible role of hepatocyte growth factor. J Stem Cell Res Ther. 2011; 1:3.
- Ruiz-Larrea M. B., Leal A. M., Liza M., Lacort M., de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron induced lipid peroxidation of rat liver microsomes. Steroids. 1994; 59:383–388.
CrossRef - Ellman G. L. Tissue sulfhydryl groups. Arch Biochem. 1959;82:70–77.
CrossRef - Moshage H., Kok B., Huizenga J. R. Nitrite and nitrate determination in plasma a critical evaluation. Clin Chem. 1995;41:892–896
- Higashino K., Takahashi Y., Yamamura Y. Release of phenylacetate esterase from liver microsomes by carbon tetrachloride. Clin Chim Acta. 1972;41:313–320.
CrossRef - Watson A. D., Berliner J. A., Hama S. Y., Du B. N. L., Faull K. F., Fogelman A. M., Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891.
CrossRef - Harvey B. H., Joubert C., du Preez J. L., Berk M. Effect of chronic N-acetyl cysteine administration on oxidative status in the presence and absence of induced oxidative stress in rat striatum. Neurochemical Res. 2008;33(3):508–517
CrossRef - Pillai A., Parikh V., Terry A. V Jr., Mahadik S. P. Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2007;41:372-86.
CrossRef - Dietrich-Muszalska A., Kontek B., Rabe-Jabłońska J. Quetiapine, olanzapine and haloperidol affect human plasma lipid peroxidation in vitro. Neuropsychobiology. 2011;63(4):197-201.
CrossRef - Thomas D. D., Miranda K. M., Citrin D., Espey M. C., Wink D. A. Nitric Oxide. In: Combat Medicine: Basic and Clinical Research in Military, Trauma, and Emergency Medicine. Tsokos G. c., Atkins J. L (Eds) Humana Press Inc., Totowa N. J. 2003;23-60.
CrossRef - Du B. N. L. Human serum paraoxonase: arylesterase. In: Kalow W., editor. Pharmacogenetics of Drug Metabolism. New York: Pergamon Press. 1992, pp.51–91.
- Ng D. S., Chu T., Esposito B., Hui P., Connelly P. W., Gross P. L. Paraoxonase-1 deficiency in mice predisposes to vascular inflammation, oxidative stress, and thrombogenicity in the absence of hyperlipidemia. Cardiovasc Pathol. 2008;17(4):226-32.
CrossRef - Wehr H., Bednarska-Makaruk M., Graban A., Lipczynska-Lojkowska W., Rodo M., Bochynska A., Ryglewicz D. Paraoxonase activity and dementia. J Neurol Sci. 2009;283(1–2):107–108.
CrossRef - Zengi O., Karakas A., Ergun U., Senes M., Inan L., Yucel D. Urinary 8-hydroxy-2’-deoxyguanosine level and plasma paraoxonase 1 activity with Alzheimer’s disease. Clinical Chemistry and Laboratory Medicine. 2011;50:529-534.
- Abdel-Salam O. M. E,, Youness E. R., Mohammed N. A., Elhamed W. A. A. Nuclear factor-kappa B and other oxidative stress biomarkers in serum of autistic children. Open J Mol Integr Physiol. 2015;5:18-27.
CrossRef - Sarandol A., Sarandol E., Acikgoz H. E., Eker S. S., Akkaya C., Dirican M. First-episode psychosis is associated with oxidative stress: Effects of short-term antipsychotic treatment. Psychiatry Clin Neurosci. 2015;69(11):699-707.
CrossRef - Brinholi F. F., Noto C., Maes M., Bonifácio K. L., Brietzke E., Ota V. K., Gadelha A., Cordeiro Q., Belangero S. I., Bressan R. A., Vargas H. O., Higachi L., de Farias C. C., Moreira E. G., Barbosa D. S. Lowered paraoxonase 1 (PON1) activity is associated with increased cytokine levels in drug naïve first episode psychosis. Schizophr Res. 2015;166(1-3):225-30.
CrossRef - Nguyen S. D., Sok D. E. Preferential inhibition of paraoxonase activity of human paraoxonase 1 by negatively charged lipids. J Lipid Res. 2004;45(12):2211–20.
CrossRef - Post A., Rücker M., Ohl F., Uhr M., Holsboer F., Almeida O. F., Michaelidis T. M. Mechanisms underlying the protective potential of alpha-tocopherol (vitamin E) against haloperidol-associated neurotoxicity. Neuropsycho. pharmacology. 2002;26(3):397-407.
CrossRef - Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658.
CrossRef - Brown G. C. Nitric oxide and neuronal death. Nitric Oxide. 2010;23:153–165.
CrossRef - Lee E. E., Eyler L. T., Wolkowitz O. M., Martin A. S., Reuter C., Kraemer H., Jeste D. V. Elevated plasma F2-isoprostane levels in schizophrenia. Schizophr Res. 2008;176(2-3):320-6.
CrossRef - Jorgensen A., Broedbaek K., Fink-Jensen A., Knorr U., Soendergaard M. G., Henriksen T., Weimann A., Jepsen P., Lykkesfeldt J., Poulsen H. E., Jorgensen M. B. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res. 2013;209(3):417-23.
CrossRef - Kropp S., Kern V., Lange K., Degner D., Hajak G., Kornhuber J., Rüther E., Emrich H. M., Schneider U., Bleich S. Oxidative stress during treatment with first- and second-generation antipsychotics. J Neuropsychiatry Clin Neurosci. 2005;17(2):227-31.
CrossRef - Miller R. Mechanisms of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics and individual variation in sensitivity to their actions Part II. Curr Neuropharmacol. 2009;7(4):315-30.
CrossRef - Xiu M. H., Chen D. C., Wang D., Zhang K., Dong A., Tang W., Zhang F., Liu L. J., Liu J. H., Liu H. B., Yang F. D., Kosten T. R., Zhang X.Y. Elevated interleukin-18 serum levels in chronic schizophrenia: Association with psychopathology. J Psychiatr Res. 2012;46(8):1093-1098.
CrossRef - Naudin J., Mège J. L., Azorin J. M., Dassa D. Elevated circulating levels of IL-6 in schizophrenia. Schizophr Res. 1996;20(3):269-73.
CrossRef - Abdel-Salam O. M. E., Youness E. R., Omara E. A., Sleem A. A. Effect of adipose tissue-derived mesenchymal stem cell treatment on oxidative stress and inflammatory response following Escherichia coli lipopolysaccharide. Comp Clin Pathol. 2015;24 (2):343–358.
CrossRef - Abdel-Salam O. M. E., Youness E. R., Omara E. A., El-Sayed., El-Shamarka M., Sleem A. A. Protection by intraperitoneal administration of bone marrow-derived stem cells of lipopolysaccharide-induced brain and liver damage in mice. Comp Clin Pathol 2016;25(1):107–118.
CrossRef - Semedo P., Correa-Costa M., Antonio Cenedeze M., Avancini M. C.,Malheiros D., dos Reis M. A., Shimizu M. H., Seguro A. C., Pacheco-Silva A., Camara N. O. S . Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27(12):3063-73.
CrossRef








