Hanan L. Al-Omary1 , Zainab M. Alawad1
, Zainab M. Alawad1 and Bassam Bernieh2
and Bassam Bernieh2
1Physiology department Medical College /Baghdad University Bab Al-Muadham - Baghdad - Iraq.
2The Heart Medical Center, Tawam Hospital Al Ain – UAE.
Corresponding Author E-mail: z_m_ch@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1394
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease with polymorphic expression. B cells have an essential contribution in immune system activation via the production of different cytokines and functioning as potent antigen-presenting cells. Therefore, a drug that particularly targets B cells may represent an ideal therapeutic approach for SLE. Rituximab (RTX), an anti-CD20 monoclonal antibody causing transient B-cell depletion, has been used to manage SLE. This study aims to assess Rituximab effects on lupus nephritis (LN) patients when added to conventional immunosuppressants. Twenty four patients, 15-32 years old, with class III/IV/V LN were involved in this study. All were on steroids before lupus nephritis occurrence. They were given rituximab induction therapy and mycophenolate mofetil (MMF) maintenance therapy. RTX was indicated for refractory and relapsing SLE. Several investigations done before and after RTX treatment and in the last follow up (done one year after starting Rituximab). Those included protein in urine, serum creatinine, double stranded DNA, C3, C4, and Estimated Glomerular Filtration Rate (eGFR). Proteinuria decreased significantly after RTX treatment and in the last measurement (P=0.01 and P=0.001, respectively). Serum creatinine significantly decreased only in the last measurement (P=0.02). Double stranded DNA decreased remarkably after treatment (P=0.01) with a further decrease in the last measurement (P=0.006). C3 and C4 increased after treatment but the increase was significant only for C3 (P=0.002) and this increase continues till the last measurement (P=0.0006). Active urine sediments found in fifteen patients and disappeared after RTX treatment. Rituximab can be promising in treating lupus nephritis when used along with traditional immunosuppressants. It can reduce disease activity and improve renal function in such patients.
Keywords
Autoimmune Disease; B-Cell Depletion; Lupus Nephritis; Monoclonal Antibody; Rituximab; SLE
Download this article as:| Copy the following to cite this article: Al-Omary H. L, Alawad Z. M, Bernieh B. Anti CD20 Monoclonal Antibody (Rituximab) as a Rescue Treatment in Severe and Refractory SLE. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Al-Omary H. L, Alawad Z. M, Bernieh B. Anti CD20 Monoclonal Antibody (Rituximab) as a Rescue Treatment in Severe and Refractory SLE. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18997 |
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease, and it is a relapsing one with polymorphic expression. Lupus nephritis (LN) is the main reason for morbidness and fatality in people affected by SLE.1
The production of autoantibodies and the generation of immune deposits in tissues can explain the pathogenic role of B cells in this disease.2 Antibodies attacking many types of self- antigens (extracellular, intracellular, cell surface-bound or soluble antigens) are common findings in people having SLE.3
Remarkable advancements have developed in the field of SLE management; those showed a great influence on the improvement of life style and the reduction of death rates in patients with SLE.4
Corticosteroids, Azathioprine (AZA), Methotrexate (MTX), Cyclophosphamide and Mycophenolate mofetil (MMF) have been the main medicines used in the last decades in treating SLE patients. Nonetheless, those have toxic effects on many tissues and organs in the body, in addition to increment of the possibility of infections and malignancies.4
Progresses in the comprehension of the immune system and the pathogenesis of SLE have helped number of researchers to seek and to discover novel immunotherapeutic strategies including treatments that influence specific immune cells such as B cells, and molecules including costimulatory molecules and cytokines, which are essential in the pathogenesis of SLE.4
A more targeted immunotherapies for instance monoclonal antibodies targeting cells (e.g. rituximab), can be used for treating patients who have not responded to standard immunosuppressants.4,5
Rituximab is a chimeric genetically engineered anti-CD20 monoclonal antibody, which depletes B cells from the peripheral circulation, but permits recreation from stem cells.6 CD 20 is a ligand existing on the developing B cells, but excluding stem cells and plasma cells5. In 1997, the Food and Drug Administration licensed Rituximab for treating relapsing or refractory, CD-20 positive, B-cell, non-Hodgkin’s lymphoma.7
As seen in some studies, Rituximab specifically diminishes CD20 mature and immature B cells, following four weekly intravenous doses and this depletion remains for more than six months after taking the drug. This happens through many different ways, which include antibody-dependent cell-mediated cytotoxicity, apoptosis and complement-mediated cytotoxicity. During Rituximab treatment, plasma cells will not be affected thus no remarkable rise in the possibility of infections happens.8’9
Many studies have recently conducted about these new biological agents such as rituximab, and their role in autoimmune diseases. However, not all of the studies have reached the same conclusion about the response of patients to these drugs. So, this study was done in order to reach more understanding regarding this issue.
The objective of this study is to assess Rituximab effects on renal function when added to conventional immunosuppressants in patients with lupus nephritis.
Material and Methods
Twenty four SLE patients with LN were involved in this prospective study that was done at Tawam Hospital in the UAE between October 2015 and November 2016. All SLE patients with lupus nephritis treated with Rituximab as a rescue treatment.
A consent was taken from the patients and the ethical committee in Tawam hospital agreed on the strategy of the research. In addition, the research was in accordance with Medical College of Baghdad University’s ethics review and approval procedures.
Patients were subjected to the following inclusion criteria: 1) diagnosis of SLE according to the American College of Rheumatology (ACR) 2) presence of follow- up to 12 months after treatment 3) active class III or IV, or class V lupus nephritis were confirmed by renal biopsy done less than 3 months prior to RTX introduction (with no contraindication).
The Indications for Rituximab treatment were:
Refractory LN to first line treatment.
Relapse while on full dosage of
Side effects to immunosuppressants.
Table 1 displays the basic characteristics of the participants including gender, median age, the classes of SLE that they have and the duration of the disease before using Rituximab.
Table 1: Characteristics of the patients at baseline.
| Patients Number | 24 |
| Gender: F/M | 21/3 |
| Age (years): Median (range) | 20 (15-32) |
| LN (WHO) classes: | |
| III | 3 |
| IV | 15 |
| IV+V | 6 |
| Duration of SLE before Rituximab (months): Median (range) | 45.5 (6 – 126) |
The WHO classes of SLE in the studied patients with the duration of SLE and the indication for the usage of Rituximab are shown in table 2.
Table 2: WHO classification, duration of SLE and the RTX indication for treatment.
| Patient | Age (years) | Sex | WHO LN class | SLE Duration (months) | RTX Indication |
| 1 | 15 | F | IV | 18 | Refractory |
| 2 | 18 | F | IV | 90 | Relapse |
| 3 | 18 | M | IV | 7 | Refractory |
| 4 | 19 | F | IV + V | 82 | Relapse |
| 5 | 21 | F | IV + V | 6 | Refractory |
| 6 | 26 | F | III | 60 | Refractory+ side effects |
| 7 | 29 | F | IV | 30 | Relapse + side effects |
| 8 | 32 | F | IV | 126 | Relapse |
| 9 | 19 | F | IV + V | 81 | Refractory |
| 10 | 18 | F | IV | 19 | Relapse |
| 11 | 15 | F | IV | 6 | Refractory |
| 12 | 29 | F | IV | 80 | Relapse+ side effects |
| 13 | 21 | M | IV + V | 7 | Relapse |
| 14 | 32 | F | IV | 122 | Refractory+ side effects |
| 15 | 17 | F | IV | 59 | Relapse |
| 16 | 32 | F | III | 32 | Relapse |
| 17 | 17 | M | IV + V | 79 | Relapse |
| 18 | 21 | F | IV | 17 | Refractory+ side effects |
| 19 | 15 | F | IV | 8 | Relapse |
| 20 | 29 | F | IV | 83 | Refractory |
| 21 | 18 | F | IV + V | 7 | Refractory |
| 22 | 32 | F | IV | 125 | Relapse+ side effects |
| 23 | 19 | F | IV | 59 | Refractory |
| 24 | 31 | F | III | 28 | Refractory |
All patients were taking corticosteroids and mycophenolate mofetil (MMF) before Rituximab treatment; eighteen of them were taking also Cyclophosphamide IV, and twelve were taking azathioprine (AZA). Cyclosporin was taken by six patients and Therapeutic plasma exchange (TPE) was done for six patients as well (Table 3).
In 50% of the patients, Rituximab was indicated due to resistant LN whereas in the other 50% relapse was the indication for its use (Table 3).
Table 3: treatment of patients before Rituximab and the indication for its usage.
| Treatment before Rituximab | Number of patients (%) |
| Corticosteroids (IV + oral) | 24 (100%) |
| Mycophenolate mofetil ( MMF) | 24 (100%) |
| Cyclophosphamide IV | 18 (75%) |
| Azathioprine (AZA) | 12 (50%) |
| Cyclosporine | 6 (25%) |
| Therapeutic plasma exchange (TPE) | 6 (25%) |
| Indications of Rituximab | Number (%) |
| Resistant SLE | 12 (50%) |
| Relapses | 12 (50%) |
After collecting the blood samples, number of investigations were done before treatment and they are shown in table 4.
Table 4: The investigations of the patients before starting Rituximab.
| Investigations | Mean ± SD |
| Proteinuria (g/l) | 4.2 ± 2.4 |
| S. Creatinine (µmol/l) | 150 ± 69 |
| e GFR EPI (ml/min/1.73 m2) | 55 ± 42 |
| double stranded DNA (ds DNA) (IU/ml) | 422 ± 262 |
| C3 (g/l) | 0.3 ± 0.12 |
| C4 (g/l) | 0.08 ± 0.03 |
| *SD= standard deviation |
After the treatment these investigations were repeated and compared with the previous ones before treatment then repeated and compared again in the last follow up.
Rituximab infusion (RITUXAN®, IDEC Pharma corp. and Genentech, inc. California, USA) was prepared by the pharmacy of Tawam hospital, and it was given as per specific infusion protocol. Antihistamine was given in all patients before starting rituximab. Rituximab dose was 500 mg four times weekly for four weeks in nine patients and 1g two times per week for two weeks in fifteen patients. It was well tolerated in all patients, with minor flu like symptoms in some of them, but no severe infections or complications were noticed.
The indicators to the outcome are Stabilization and/ or improvement in the following parameters:
Clinical manifestations
Creatinine
eGFR (EPI)
Proteinuria
Urine sediment
ds DNA titer
C3, C4 levels
All data were tabulated using Microsoft Excel and statistical analysis was done using SPSS 17.0. All values were in mean ± SD and using unpaired t tests where appropriate. A P value of less than 0.05 was considered significant.
Results
Comparing serum creatinine before the treatment with Rituximab with its value after the treatment, it was found that there is a non- significant reduction. The e GFR increased after the therapy, but the increase was not significant (table 5).
Table 5: Comparison of serum creatinine, e GFR, proteinuria, double stranded DNA, C3 and C4 before and after treatment with RTX.
| Investigation | Pre RTX Mean± SD | Post RTX Mean± SD | P Value |
| S. Creatinine µmol/l | 150 ± 69 | 82 ± 53 | 0.06 |
| e GFR (EPI) ml/min/1.73 m2 | 55 ± 42 | 97 ± 37 | 0.08 |
| Proteinuria g/l | 4.2 ± 2.4 | 2 ± 0.95 | 0.01 |
| Double stranded DNA IU/ml | 422 ± 262 | 108 ± 72 | 0.01 |
| C3 g/l | 0.3 ± 0.12 | 0.9 ± 0.4 | 0.002 |
| C4 g/l | 0.08 ± 0.03 | 0.17 ± 0.12 | 0.05 |
| *SD= standard deviation |
The amount of proteinuria and the double stranded DNA decreased after the treatment with a significant difference (P=0.01 for each).
C3 and C4 were also compared and showed a significant increase regarding C3 (P=0.002) and a high but non-significant increase in C4 (P=0.05) (table 5).
Comparing proteinuria before and after the treatment of RTX, it is obvious that there is significant decrease after treatment and there is a more significant decrease in the last follow up measurements (figure 1).
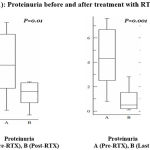 |
Figure 1: Proteinuria before and after treatment with RTX.
|
Regarding C3, it was found that there is a significant increase after treatment and in the last follow up measurement (figure 2).
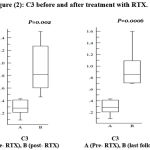 |
Figure 2: C3 before and after treatment with RTX.
|
While C4 shows an increase after treatment with no significant difference and it starts to decrease during the last follow up measurements (figure 3).
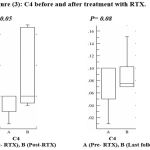 |
Figure 3: C4 before and after treatment with RTX.
|
The amount of double stranded DNA decreased significantly after treatment and measuring it in the last follow up showed a further significant decrease (figure 4).
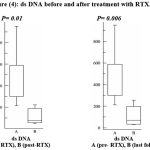 |
Figure 4: ds DNA before and after treatment with RTX.
|
Serum creatinine on the other hand showed a non- significant decrease after treatment and a significant decrease in the last follow up (figure 5).
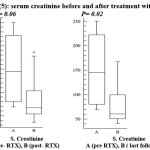 |
Figure 5: serum creatinine before and after treatment with RTX.
|
Measuring eGFR after treatment showed no significant increase from that before treatment and in the last follow up the increase was also not significant (figure 6).
 |
Figure 6: eGFR before and after treatment with RTX.
|
The active urine sediment was found in fifteen patients before treatment and it was none after treatment.
The time taken to respond to the treatment was (median 8 weeks, range 4- 12 weeks). The clinical manifestations improved in symptomatic patients as 21 participants (87.5%) responded to Rituximab. Only three patients with class IV+ crescents did not respond and evolved to End stage renal disease (ESRD) in 6 months. Also, three patients had severe relapse 1 year after starting Rituximab due to poor compliance.
Discussion
Recently, B cell depletion therapy has been assessed in people having various autoimmune diseases such as SLE.
In the present study, the therapeutic effects of anti CD20 monoclonal antibody (Rituximab) were investigated in patients having SLE with lupus nephritis.
Proteinuria is noticed to decrease significantly after treatment, which goes with what was found by Vigna-Perez et al11 in 2006, who reported that there was remarkable decrease in disease activity (p< 0.05), and proteinuria (p< 0.05) after two months and then after three months of Rituximab therapy.
In severe lupus nephritis, a significant improvement in patient’s renal survival was found in cases of partial remission in comparison to cases without remission.12
It was shown that some patients have inadequate response after Rituximab treatment and it was noticed that polymorphisms of the FcγRIIIa gene have an essential role in the effect of B cell depletion therapy. So, the clinical response of patients affected by autoimmune diseases treated with rituximab may depend on FcγRIIIa genotype.13
Our study showed a decrease in serum creatinine in the last follow up measurement, and this agrees with a study that reported improvement of renal function after the beginning of Rituximab treatment.14
The complement system (a group of plasma and membrane proteins) has substantial value in SLE. Many proteolytic reactions are responsible for its activation in order to protect against infections. It participates in the inflammatory reactions and tissue injury in lupus erythematosus. However, insufficiency of some complement proteins can significantly increase the susceptibility to SLE. In addition, complement deficiency may affect normal B-cell tolerance thus result in formation of autoantibodies.15
In this study, C3 was found to increase significantly after treatment, but the elevation of C4 was non- significant. This was also reported by other studies that observed a significant improvement in C3 from the time of B-cell depletion to 6 months following the therapy.16,17 Other researchers reported that Rituximab did not change complement levels in SLE cases included in their studies.11
Double stranded DNA showed significant decrease after treatment and this reduction continues till the last follow up time in this study. This was also approved by another study which noticed a prolonged decline in anti-dsDNA antibodies in SLE patients which lasted for more than one year after treatment18 and a study that collected data from 20 studies of 300 SLE participants and these studies showed reduction of anti-dsDNA antibodies and good response rate (about 75%) to Rituximab.19 The decrease in anti-dsDNA antibodies was also evident in children with refractory SLE treated by Rituximab.17
It was observed that disease reactivation in SLE happened only following B-cell repopulation. Rituximab results in lysis of B cells done by complement and by Fc-receptor-bearing cytotoxic cells, and it also leads to apoptosis. Rituximab has a range of effects on immunoglobulin levels with variable decrease of autoantibody titers; it is probable that rituximab has other actions on the immune system that results in improvement of serum C3 levels and decrease of anti-dsDNA antibodies and B-cell depletion which lasted for 3 to 11 months.20
Glomerular filtration rate (GFR) increased after treatment, however, the rise was not significant in this research. This was also observed by other researchers who reported a slight increase in GFR or an increase of about 25%.21 The non- responders had longer period of nephritis and lower GFR than the patients with full or limited responses and the variable response to Rituximab between the patients may be attributed to the development of human anti-chimeric antibody (HACA) titers that associated with poor B cell depletion.22
The main limitation of our study is the relatively small sample size. So, it is recommended to increase the number of the participants in future studies and to do more studies including randomized controlled trials to reach more understanding about the uses and the effects of Rituximab and the mechanism behind the low response to this medicine in some patients with Lupus nephritis.
Conclusion
Contemporary treatment of severe lupus nephritis is unsatisfactory regarding the outcome and the toxicity. Rituximab is a promising drug in patients having lupus nephritis resistant to the standard immunosuppressive therapy. Rituximab causes a remarkable decrease in disease activity in patients with severe LN when it is used in conjunction with the traditional immunosuppressants.
Acknowledgment
We are grateful to the nurses, staff and pharmacy of Tawam Hospital in Al-Ain, the UAE, for their care. We thank the nephrology doctors in the hospital for their patient referrals. Also we acknowledge the help of the lab staff for their assistance.
Conflicts of interest
None of the authors of this paper have any conflict of interest.
Funding source
Self-funded by the researchers.
References
- Pepper R, Griffith M, Kirwan C, Levy J, Taube D, Pusey C, et al. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Dial. Transplant. 2009;24:3717-23.
CrossRef - Chan O, Madaio M.R, Shlomchik M.J, Chan O.I, Madaio M.P. The central and multiple roles of B cells in lupus pathogenesis. Rev. 1999;169:107-21.
CrossRef - Vassilopoulos D and Hoffman G. S. Clinical utility of testing for antineutrophil cytoplasmic antibodies. Diagn. Lab. Immunol. 1999;6:645-51.
- Karim M. Y, Pisoni C. N, Khamashta M. A. Update on immunotherapy for systemic lupus erythematosus—what’s hot and what’s not! 2009;48:332-41.
- Looney R. J, Anolik J. H, Campbell D, Felgar R. E, Young F, Arend L. J, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose‐escalation trial of rituximab. Rheum. 2004;50:2580-9.
CrossRef - Leandro M. J, Edwards J. C, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Rheum. Dis. 2002;61:883-8.
CrossRef - Grillo-López A. J, White C. A, Varns C, Shen D, Wei A, McClure A, et al. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Oncol. 1999;26:66-73.
- Catapano F, Chaudhry A. N, Jones R. B, Smith K. G, Jayne D. W. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Dial. Transplant. 2010;25:3586-92.
CrossRef - Eisenberg R. SLE-Rituximab in lupus. Res. Ther. 2003;5:157- 159.
- Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, Portales-Pérez D, Baranda L, Abud-Mendoza C, et al. Clinical and immunological effects of Rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Res. Ther. 2006;8: 83.
- Chen Y. E, Korbet S. M, Katz R. S, Schwartz M. M, Lewis E. J. Collaborative Study Group. Value of a complete or partial remission in severe lupus nephritis. J. Am. Soc. Nephrol. 2008;3:46-53.
CrossRef - Anolik J. H, Campbell D, Felgar R. E, Young F, Sanz I, Rosenblatt J, et al. The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Rheum. 2003;48:455-9.
- Podolskaya A, Stadermann M, Pilkington C, Marks S. D, Tullus K. B cell depletion therapy for 19 patients with refractory systemic lupus erythematosus. Dis. Child. 2008; 93:401-6.
CrossRef - Sozeri B, Mir S, Berdeli A. Complement-4 deficiency in a child with systemic lupus erythematosus presenting with standard treatment-resistant severe skin lesion. Rheumatol. 2011;2011:1-5.
CrossRef - Gillis J. Z, Dall’Era M, Gross A, Yazdany J, Davis J. Six refractory lupus patients treated with rituximab: a case series. Care. Res. 2007;57:538-42.
CrossRef - Marks S. D, Patey S, Brogan P. A, Hasson N, Pilkington C, Woo P, Tullus K. B lymphocyte depletion therapy in children with refractory systemic lupus erythematosus. Rheum. 2005;52:3168-74.
CrossRef - Cambridge G, Leandro M. J, Teodorescu M, Manson J, Rahman A, Isenberg D. A, et al. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Rheum. 2006;54:3612-22.
CrossRef - Jayne D. Role of rituximab therapy in glomerulonephritis. Am. Soc. Nephrol. 2010; 21:14-7.
CrossRef - Leandro M. J, Cambridge G, Edwards J. C, Ehrenstein M. R, Isenberg D. A. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. 2005;44:1542-5.
- Lindholm C, Börjesson-Asp K, Zendjanchi K, Sundqvist A. C, Tarkowski A, Bokarewa M. Longterm clinical and immunological effects of anti-CD20 treatment in patients with refractory systemic lupus erythematosus. Rheumatol. 2008;35:826-33.
- Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann. Rheum. Dis. 2008;67:1724-31.
CrossRef








