Asep Sukohar1 , Hening Herawati2, Hendra T. Sibero3, Setiawan Gigih4
, Hening Herawati2, Hendra T. Sibero3, Setiawan Gigih4 , Risti Graharti4
, Risti Graharti4 , Wahyudo Riyan4
, Wahyudo Riyan4  and Morfi Chicy Widya4
and Morfi Chicy Widya4
1Department of Pharmacology and Therapy Medical Faculty of Lampung University, Lampung 35145, Indonesia.
2Department of Research and Development, Dharmais Cancer Hospital, Jakarta 11420, Indonesia.
3Department of Dermatology, Faculty of Medicine, Lampung University, Lampung 35145, Indonesia.
4Faculty of Medicine, Lampung University, Lampung 35145, Indonesia.
Corresponding Author E-mail: graharti@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1391
Abstract
The development of anticancer drugs from nature’s ingredients are very attracted for world researchers. Caffeine contained in coffee has long been scrutinized as anticancer hepar, in vitro researched on hepatocelluler cancer cell (HCC) and rats. HCC is one of five vicious cancers in the world, requiring better management and early detection. Micro RNA (mir) is a nucleotide composing 19-20 pairs of bases that can be used as diagnostic, therapeutic and preventive or early detection of cancer. We have examined the expression of miRNA 146 A, mir-103, 423-3p, 16, 21, and in this study, we focus on the mir-423-3p which are treated with 0.5 mM caffeine. The purpose of this research was to assess the influence of caffeine 0.5 mM against Hep-G2 cells by assessing expression of mir-423-3p. The study was carried out using invitro cell Hep-G2, with 30 groups of sample consist of 15 sample controls and 15 samples treated 0.5 mM caffeine, using qPCR CFX-96 type and expression of mir-423-3p analyzed by the Livaks method. Expression of mir-423-3p examined at 0, 2, 8, 18 and 24. There is a variation in the expression of mir-423-3p from 0 hours to 24 hours after treating caffeine 0.5 mM. Expression of mir-423-3p is lowest in 8 and 18 hours after treating caffeine 0.5 mM (0.11), the highest expression is at 0 hours(0.26) and 24th hour (0.17). Caffeine decreased expression of mir-423-3p.
Keywords
Caffeine; Expression of mir-423-3p; Hep-G2
Download this article as:| Copy the following to cite this article: Sukohar A, Herawati H, Sibero H. T, Gigih S, Graharti R, Riyan W, Widya M. C. Effects of Caffeine Againts Expression on Mir-423-3p in Cell Lines Hep-G2. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Sukohar A, Herawati H, Sibero H. T, Gigih S, Graharti R, Riyan W, Widya M. C. Effects of Caffeine Againts Expression on Mir-423-3p in Cell Lines Hep-G2. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18939 |
Introduction
Coffee contains a wide range of chemical components with different characteristics, but there are still many unknown biological activity and its benefits to mankind in coffee compounds.1 Coffee consists of volatile and non-volatile compound that affect the aroma and quality of coffee.
Another content of coffee is caffeine which includes xanthin alkaloid compounds of polyphenols as an antioxidant activity. Polyphenol in robusta coffee is higher than Arabic coffee or other plants.2 Caffeine in coffee has a strong antioxidant activity, toxic to Artemia selina, inhibits cell proliferation of liver cancer cells and preventing/reducing the incidence of liver cancer in rats.3,4
Antioxidants are divided into three groups, namely the phenol, the amin and the amino-phenols.5 Caffeine inhibits the growth of Hepatocellular Cancer Cell (HCC) through the mechanism of apoptosis and cycles of G0/G1. Caffeine activates regulatory kinase (MEK), which is responsible for inducing epidermal growth factor receptor (EGFR).6 Caffeine can inhibit growth and kill HCC cell with Hep-G2 model, despite in large concentrations.6
Hepatocellular (HCC) is a type of solid tumors which often found in the world and the incidence increased from year to year. We need management improvement efforts for HCC for the development of therapies derived from natural ingredients, prevention and the early detection.6 Early detection of cancer needs to do to prevent complications for a better life.
There are several methods for the early detection of cancer through examination of HCC, including, alpha fetoprotein, method of Ye JZ and Mei-Sze Chua.7 Besides alpha fetoprotein, method of Ye JZ and Mei-Sze Chua, early detection of cancer can be assessed by micro-RNA gene expression (miRNA/mir).8 Micro RNA (miRNA/mir) is an ribonukleotida acid and non-protein-coding, very small sized 19-25 base pairs, play a role in gene regulation.9 Gene expression is the process of delivering information from DNA and can be copied by the transcription process in eukaryotic organisms. In addition, any translational effect on gene expression. Gene expression largely controlled at the level of transcription. The transcription factors bind to the promoter that will determine those genes to be transcribed. However, gene expression can also be controlled at the level of translation such as the role of miRNA to 3’UTR mRNA.9-13 Target mir 423-3p are a mir 253 and 2 potential targets that still need to be examined, namely the AdipoR2 (adiponectin receptor 2) and DUSP4 (dual specificity phosphatase 4). Mechanism of target mir-423-3p against mir 253, DUSP4 and adipoR2 still unknown clearly.14
In addition to diagnostic and prognostic functions mir as therapy, there are 2 types of mir 423, 423-3p and 423-5p. This research used mir 423-3p which can be expressed on cell Hep-G2 series 1886 and PLC5.15,16 This research provides information that caffeine inhibites the growth of cells Hep-G2 and affects gene expression mir-423-3p.
Materials and Methods
The study was conducted at the Department Of Molecular Biology Dharmais Cancer Hospital by using cell Hep-G2 series: 1886. Hep-G2 cell culture using medium (DMEM/F12, Gibco) containing 10% Fetal Bovine Albumin (Sigma) and the antibiotic penicillin-streptomycin (100 mg/L), the number of cells per well 0,5×104 grown in 96-well and with 0.1% DMSO concentration and analyzed using Elisa Reader. Caffeine used obtained from Sigma-Aldrich catalog number Jakarta: C0750.
The study was done invitro and was divided into two groups, namely the group of control and treated groups which were given caffeine 0.5 mM. Administering caffeine was performed in 48 hours after cell culture Hep-G2 and confluent 60-80%. The treatment was carried out three times, including for the control group.
Isolation of total RNA used Exiqon miRCURY ™, RNA Isolation Kit Product Code-300110 from Exiqon. Making cDNA used cDNA synthesis kit Universal product code 203300 from Exiqon. Primary mir-423-3 p (product code number Exiqon 204488) was obtained from the BioRad through PT.Scienwerke.
Synthesis of cDNA was carried out in a total volume of 20 μl consisting of: a) 5 x Reaction buffer: 4 l, Nuclease-free water 9: l; b) Enzyme mix 2: l; c) Synthetic spike in with H2O 1: l; d total RNA Template) (5 ng/µl) 4: l. Incubation was performed for 60 minutes at a temperature of 42˚C, followed by reverse transcriptase for 5 minutes at a temperature of 95˚C, then immediately cooling at 4°C and stored at a temperature 4°C or in the freezer.
RT-PCR amplification was carried out with a total volume of 10 μl consisting of: a) Sso EvaGreen supermix 5 μl Fast; b) Primary (forward and reverse) 2 μl; c 2 μl cDNA templates), and d) 1 μl H2O. Do denaturation 95°C for 10 minutes, the amplification of 60°C for 10 seconds, followed by a decrease in temperature of up to 10°C for 1 minute. Each cycle consists of a total of 40 cycles.
Expression of mir-423-3p was examined with RT-PCR CFX-96 at 0, 2, 8, 18 and 24 hours after treating caffeine, then the value of the expression was compared between groups treated and group control. Data was evaluated by Livaks method and statistically with repeated measurement. Analysis of gene expression mir-423-3p using Livak method with formula: 2-∆Cq. Normalization was done by subtracting each value Cq target before and after treating (based on 5 time difference) with each value of the Cq reference so obtained values of ∆Cq.17
Discussion
Caffeine can be found in many popular beverages, including cocoa, tea and coffee. Although caffeine is most commonly used as a stimulant to prevent sleepiness and as a remedy for pain, there is a mounting body of in vitro evidence suggesting that caffeine inhibits the growth of a variety of cancer cells through cell cycle arrest and the induction of apoptosis and that caffeine enhances the toxicity of radiation and chemotherapeutic drugs.18-20 In clinical settings, high consumption of caffeine has been associated with beneficial effects on the liver, including a lower incidence of chronic liver disease and a reduced risk of HCC.21,22 However, the molecular mechanisms which caffeine exerts beneficial effects on HCC are poorly defined.21
We demonstrated that treatment with caffeine at 0,5 mM concentrations inhibited the growth of HCC cells. Finally, we used 0,5 mM for our study as showing in figure 1.
The data result of caffeine cytotoxicity test against Hep-G2 cells was expressed in percent of cell death shows in figure 1. The inhibition growth Hep-G2 50% occurred in 24 hours after administering caffeine 0.5 mM. A different study was done by Jun-ichi Okano et al in 2007 that a dose of caffeine inhibit the growth of cells Hep-G2 were 1-20 mM. Even so, there are similarities that caffeine is chemo-preventive and inhibited the growth of cells Hep-G 2.6 The Inhibition growth of Hep-G2, can be observed through the variation of time after treating caffeine 0.5 mM and assessed based on the value of Cq 2. The smaller value of Cq in one thermal cycle is become more usefulclues of Hep-G2 were alive. The value of Cq before treated caffeine in figure 2 as follows; the hours of 0, 2, 8, 18 and 24 are consecutive (30.57), (29.83), (29.51), (29.18), (29.17). The value of Cq after treating caffeine in figure 2 as follows; the hours of 0, 2, 8, 18 and 24 were (32.51), (31.88), (32.35), (32.02) and (31.69).
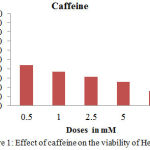 |
Figure 1: Effect of caffeine on the viability of Hep-G2
|
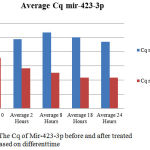 |
Figure 2: The Cq of Mir-423-3p before and after treated caffeine based on differenttime
|
The difference value can be determined based on the value of the expression of mir-423-3p using the formula Livaks.17
The gene expression can be detected from RNA or proteins and can be quantitatively as well as qualitatively. The examination of the expression of mir-423-3p on this research using cDNA obtained from RNA isolation Protocol Exiqon, using the miRCURY ™ RNA Isolation Kit Product Code-300110 from Exiqon. Examination of gene expression of mir-423-3p was done by RT-PCR machine with software CFX-96.
Gene activity of mir-423-3p is measured based on gene expression by the relatifitas theory with the basic principle Livak method with formula 2-—dCT.17 The results analysis inaccordance at Figure 3 shows information that caffeine is capable for influencing the activity of mir-423-3p by comparing the time at 0, 2, 8, 18 and 24 hours between before and after treating. The expression of mir 423-3p is highest at the 0 and the lowest at 8 and 18 hours. At the 24th hour the expression of mir- 423-3p began to increase (0.17) as appears in Figure 3. These data provide information that caffeinecan inhibit/kill cells Hep-G2 with active period (duration of action) less than 24 hours.
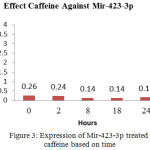 |
Figure 3: Expression of Mir-423-3p treated caffeine based on time
|
The value of miRNA 146 A expression against mir-423-3p on the previous study (0.05) 15 was smaller than the value of the mir-423-3p expression treating caffeine 0.5 mM. Differences in the expression of value caused by caffeine which is capable inhibiting/killing cells Hep-G2. However, mir-423-3p is expressed on cell Hep-G2 1886 and PLC5.15
The value of mir-423-3p expressionon cell larynx cancer and Hep-G2 respectively (1.5) and (3.0).14 There is a difference between the expression value of some researchers, this is due to different methods of intervention. Although there were differences between the value of mir-423-3p expression,clearly that mir-423-3p expressed on normal human cells hypopharyngeal cells (NHPs), Hep-G2 series 1886 and PLC 5.14,15
Caffeine has been reported to affect cell cycle function and to induce apoptosis in pancreatic cancer and neuroblastoma cells.23 However, the growth inhibitory effect of caffeine on HCC cells was associate with cell cycle arrest alone, not apoptosis. The molecular mechanisms which caffeine inhibits cancer cell growth may be distinct depending on the cell types. The exact molecular targets of caffeine-mediated cell cycle regulation need to be further clarified, but may include cyclins and cyclin- dependent kinases. Escalating doses of caffeine activates two MAPKs, MEK/ERK1/2 and p38MAPK, in HepG2 cells, a subset of HCC cells. Because these MAPKs have often been associated with growth modulation of cancer cells, including HCC cells, in positive or negative manners depending on the cellular context.24
Mir-423 has been reported up-regulation in hepatocellular carcinoma, but only mir-423-3p contributes to the promotion of cell growth and cell cycle progression, particularly at the G(1)/S transition in hepatocellular carcinoma cells. p21Cip1/ Waf1 has been identified as a downstream target of mir-423-3p. Therefore, over-expression of mir- 423 promotes hepatic carcinogenesis through the suppression of tumor suppressor p21Cip1/Waf1 expression.14,25
AdipoR2 was identified as a target for mir-423-3p. Adiponectin is an adipocyte-derived cytokine that plays an important role not only in lipid and glucose metabolism but also in the progression of cancer. It has been shown that adiponectin may have anti-cancerous effects by suppressing tumor proliferation and promoting apoptosis. Recent studies demonstrated the antiangiogenic and tumor growth inhibiting properties of adiponectin.26,27
Adiponectin binds to two major receptors, AdipoR1 and AdipoR2. Ensuing intracellular signalling pathways link adiponectin with carcinogenesis, with the effect of stimulating AMP-activated protein kinase (AMPK), nuclear factor-κB (NF-κB), peroxisome proliferators-activated receptor (PPAR)-α and mammalian target of rapamycin (mTOR). Accumulating evidence indicates that adiponectin measurements may serve as a useful prognostic screening tool for early detection of obesity related cancers. The expression of adiponectin receptors in tumor tissues has also been elucidated. AdipoR1 and AdipoR2 were downregulated in human gastric cancer, endometrial adenocarcinoma. Knockdown of AdipoR1 and AdipoR2 relieved the suppressive effects of adiponectin on the growth of colon cancer cells. Furthermore, expression of AdipoR2 was inversely associated with T category in oesophageal cancer. Decreasing transcriptional and protein expression of AdipoR2 in laryngeal cancer cells as well as in archival human laryngeal cancer tissues, providing a translational corroboration between miR-423-3p and AdipoR2.28-30
Conclusion
In summary, we have shown that mir-423- 3p plays a novel oncogenic role in Hep-G2, whereby expression mir-423-3p is affected by concentration of caffeine and caffeine inhibit growth Hep-G2.
Acknowledgement
The authors gratefully acknowledge Prof. Dolara, DR Cristina Luceri from department pharmacology Florence University Italy, Prof. Sahidin from Haluoleo University Indonesia, Cancer Team of Dharmais Hospital Jakarta Indonesia and Sciencewerke company for continued technical assistance. This study was supported by Health Professional Education Quality Project, Indonesia Ministry of Education [Grants 2012-089/3861/UN26/KU/2012]
Conflict of Interest
There is conflict of interest
References
- Corby K . Caffeine and Decaf. in: The Joy of Coffee. New York: Houghton Mifflin. 2003;160-65.
- Johnston K.L, Clifford M.N, Morgan L.M. Coffee acutely modifies gastrointestinal hormon secretion and glucose tolerance in human. In: Glycemic effect of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78(4):728-33.
CrossRef - Sukohar A.S,Wirakusumah F.F, Sastramihardja H.S. Isolationand Characterization Cytotoxic Compounds Caffeine and Chlorogenic Acid Seed of Lampung Coffee Robusta. J Med Planta. 2011;1(4):11-26.
- Johnson S, Koh W.P, Wang R, Govindarajan S, Yu M.C, Yuan J.M. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer.Causes Control. 2011;22(3):503–10.
CrossRef - Yen G.C, Chen H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric .Food Chem. 1995;43:27-32.
CrossRef - Okano J.I, Nagahara T, Matsumoto K and Murawaki Y. Caffeine Inhibits the Proliferation of Liver Cancer Cells and Activates the MEK/ERK/EGFR Signalling Pathway. Basic Clin Pharmacol Toxicol. 2008;102(6):543-51.
CrossRef - Ye J.Z. Method and compositions for detection of liver cancer. US. 2005;200(50):202 485.
- Sukohar A, Herawati H, Witarto A.B,Wirakusumah S. F.F, Sastramihardja H.S. Roleof Chlorogenic Acid From Lampung Robusta Coffee Against Gene Expression of Mirna 146 A on Hepatocellular Carcinoma Cells. International Journal of Research in Pharmaceutical and Nano Sciences. 2013;2(6):776–84.
- Wang D, Qiu C, Wang H.Z.J, Cui Q, Yin Y. Human Micro RNA Oncogenes and Tumor Suppressors Show Significantly Different Biological Patterns: From Functions to Targets. Plos One. 2010;5(9):1-7.
CrossRef - Chendrimada T.P, Gregory R.I, Kumaraswamy E, Norman J, Cooch N, Nishikura K and Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436(7051):740-44.
CrossRef - Aaron J, Schetter A.J, Heegaard N.H.H and Curtis C.H. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. 2009;31(1):37-49.
- Liang Y, Ridzon D, Wong L and Chen C. Characterization of microRNA expression profiles in normal human tissues. Biomed Central. 2007;8(166):1-20.
CrossRef - Mignone F, Gissi C, Liuni S, and Pesole G. Untranslated regions of mRNAs. Biomed Central. 2002;3(3):1-10.
- Guan G.F, Zhang D, Zheng Y, Wen L, Yu D, Lu Y, Zhao Y. MicroRNA-423-3p promotes tumor progression via modulation of AdipoR2 in laryngeal carcinoma. Int .J. Clin Exp Pathol. 2014;7(9):5683-91.
- Sukohar A, Herawati H, Witarto A.B, Sibero H.T, Sutyarso. Comparison of Genes Expression; miRNA 146 A, mir-103, mir-423-3p, mir-21, mir-16 In Cell Lines Hep-G2 Series 1886 and PLC5. Internatinal Journal of Pharmacy and Pharmaceutical Science. 2015;7(2):76-79.
- Sukohar A, Herawati, Arief B.W, Sibero H.T, Firman S F,Wirakusuma, Herry S. MIR-423-3P Used As Reference Genefor Mirna 146 Ain Cell Lines Hep-G2. Internatinal Journal of Pharmacy and Pharmaceutical Science.2014;6(8):553-57.
- Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-08.
CrossRef - Hashimoto T, He Z, Ma W.Y, Schmid P.C, Bode A.M, Yang C.S et al. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004;64:3344–9.
CrossRef - He Z, Ma W.Y, Hashimoto T, Bode A.M, Yang C.S, Dong Z. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 2003;63:4396–401.
- Asaad N.A, Zeng Z.C, Guan J, Thacker J, Iliakis G. Homologous recombination as a potential target for caffeine radiosensitization in mammalian cells: reduced caffeine radiosensitization in XRCC2 and XRCC3 mutants. Oncogene. 2000;19:5788–800.
CrossRef - Ruhl C.E, Everhart J.E. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. 2005;129:1928–36.
- Gallus S, Bertuzzi M, Tavani A, Bosetti C, Negri E, Vecchia L.C et al. Does coffee protect against hepatocellular carcinoma. Brit J Cancer. 2002;87:956–59.
CrossRef - Jang M.H, Shin M.C, Kang I.S, Baik H.H, Cho Y.H, Chu J.P et al. Caffeine induces apoptosis in human neuroblastoma cell line SK-N-MC. J Korean Med Sci. 2002;17:674–78.
CrossRef - Okano J, Nagahara T, Matsumoto K, Murawaki Y. The growth inhibition of liver cancer cells by paclitaxel and the involvement of extracellular signal-regulated kinase and apoptosis. Oncol Rep. 2007;17:1195–2000.
CrossRef - Lin J .H.S, Wu S, Ding J, Zhao Y, Liang L, Tian Q, Zha R, Zhan R, He X. MicroRNA-423 promotes cell growth and regulates G(1)/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis. 2011;32:1641-47.
CrossRef - Kelesidis I, Kelesidis T and Mantzoros C.S. Adiponectin and cancer: a systematic review. Br J. Cancer. 2006;94:1221-25.
CrossRef - Yamauchi N, Takazawa Y, Maeda D, Hibiya T, Tanaka M, Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T and Fukayama M. Expression levels of adiponectin receptors are decreased in human endometrial adenocarcinoma tissues. Int J Gynecol Pathol. 2012;31:352-57.
CrossRef - Otani K, Kitayama J, Kamei T, Soma D, Miyato H, Yamauchi T, Kadowaki T and Nagawa H. Adiponectin receptors are downregulated in human gastric cancer. Gastroenterol . 2010;45:918-27.
CrossRef - Hiyoshi M, Tsuno N.H, Otani K, Kawai K, Nishikawa T, Shuno Y, Sasaki K, Hongo K, Kaneko M, Sunami E, Takahashi K, Nagawa H, Kitayama J. Adiponectin receptor 2 is negatively associated with lymph node metastasis of colorectal cancer. Oncol Lett. 2012;1(3):756-60.
CrossRef - Howard J.M, Cathcart M.C, Healy L, Beddy P, Muldoon C, Pidgeon G.P and Reynolds J.V. Leptin and adiponectin receptor expression in oesophageal cancer. Br. J. Surg. 2014;101:643-52.
CrossRef







