Musvi S. Altaf¹, Syed Arshad Hussain1, Chauhan Rayaz Ahmad², Willayat M. M¹, Imtiyaz A. H.³ and Shakoor Bhat¹
1Depatment of Veterinary Public Health, Skuast, India.
²Private Field Vet.
³Department of LPT, Skuast, India.
Abstract
The present investigation pertains the starch adulteration of raw milk, along with study of prevalence and toxigenicity studies of Bacillus cereus emetic strain isolates of raw milk in Kashmir valley. The investigation was conducted during October 2009-June 2010. A total of 175 samples comprising of 50 raw milk were tested. Starch/rice flour was detected in 7, samples of raw milk. Bacillus cereus emetic strains were isolated from 8 of the raw milk samples making a prevalence of 16 percent contamination of Bacillus cereus emetic strain in raw milk available in and around the Srinagar city. Different areas of the Srinagar city differed with respect to starch adulteration as well as contamination of Bacillus cereus emetic strains. Central zone had the highest percentage of positive samples of milk (20%). The field isolates and the standard strain of Bacillus cereus had similar cultural, morphological and biochemical characteristics.
Keywords
Raw milk; Bacillus cereus emetic strains; Prevalence; standard strain (NCTC 11143)
Download this article as:| Copy the following to cite this article: Altaf M . S, Hussain S. A, Ahmad C. R, Willayat M. M¹, Imtiyaz A. H, Bhat S. A Study on the Prevalence of Bacillus cereus Emetic Strains in Raw Milk in and around Srinagar City of J&K. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Altaf M . S, Hussain S. A, Ahmad C. R, Willayat M. M¹, Imtiyaz A. H, Bhat S. A Study on the Prevalence of Bacillus cereus Emetic Strains in Raw Milk in and around Srinagar City of J&K. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1876 |
Introduction
Foods of animal and plant origin primarily being the main source of nutrients, also act as vehicles for many food borne infections and intoxications, as they provide an excellent media for growth and perpetuation of micro-organisms. Despite improved levels of hygiene and sanitation in handling and preparation of food items, the incidence of food-borne infections is increasing, owing to better reporting and diagnostic techniques, changes in eating habits and identification of new human pathogens (Creraret al ., 1996).
Among the various food borne pathogens, Bacillus cereus, a gram positive, spore forming bacterium is wide spread in environment (soil, water and dust), and easily contaminates to foods of both plant and animal origin such as cereals, vegetables, milk and milk products, meat and meat products etc. there by causing food borne illnesses in humans (Larsen and Jorgeusen, 1996).
Outbreak of food poisoning due to Bacillus cereus have been described since the beginning of the last century with the first confirmed report in Norway in 1948 (Hauge, 1955). Since then many food-borne outbreaks were reported (Lund et al., 2000, Hussainet al., 2007). Milk, being ideal medium for growth of microorganisms, makes it suitable for multiplication of B. cereus as well as elucidation of its toxin in it. With the advent of increased number of psychrotolerantB. cereus strains, the dairy industry has witnessed increased reports of food poisoning outbreaks due to this organism (Granum et al., 1993).
Two distinct types of gastrointestinal disorders caused by B. cereus in humans viz; an early “emetic syndrome” and a late onset “diarrheal syndrome” involving two different types of enterotoxins, have been recognized (Kramer and Gilbert, 1989). The emetic syndrome, a food borne intoxication, caused by preformed B. cereus emetic enterotoxin (BCEET) in food has a rapid onset (1-5 hours) characterized predominantly by nausea and vomition, resembling closely to staphylococcal food poisoning (Adams and Moss, 2007). In contrast the diarrhoeal syndrome is caused due to production of B. cereus diarrhoeal enterotoxins (BCDET) during the vegetative growth of bacteria in the foods or in the intestines following ingestion, has a longer incubation period (12-24 hours) and is characterized by symptoms like diarrhoea, abdominal pain and rectal tenesmus, resembling closely Clostridium perfringens type A food poisoning (Kramer and Gilbert, 1989, Adams and Moss, 2007).
Apart from gasteroenteritisB. cereus isalso involved in a variety of non-GIT infections like meningitis, endopthalmitis, endocarditis, periodonitis, osteomyelitis, wound infection and septicaemia in humans (Schoeni and Wong, 2005). It is also emerging as potential pathogen of serious concern in animals owing to increased reports of its role in diseases like, osteomyelitis, middle ear infections, abortions and mastitis (Schieferet al. 1976). These reports unfold its explosive pathogenic role in various infections of animals.
The biological effects of B. cereus toxins have been studied extensively. The diarrhoeal enterotoxin produced during the exponential growth phase of the organism is destroyed at 56oC in 20 minutes, a temperature which is far less than the temperature attained in conventional cooking. However, emetic enterotoxin, commonly called as cerulide, produced in the stationary growth phase, is highly heat stable (126oC for 90 minutes), withstanding extremes of pH (2-11) and is unaffected by the temperature attained during conventional cooking process. As a result the episodes of food poisoning out breaks due to B. cereus emetic toxin outnumber the diarrhoeal ones.
The production of enterotoxins by B. cereus is dictated by the type of food rather than the strain involved. Studies on food ingredients have indicated rice and rice flour containing a high percentage of B. cereus emetic strains whereas diarrhoeal strains are found in almost all foods of animal origin. During growth, harvesting, milling, and other agricultural operations rice can variabily become contaminated with B. cereus spores from a wide variety of environmental sources including soil, dust, sediment, and water (Johnson et al ., 1984). The spores survive normal cooking temperatures (Vijaylakshmiet al., 1981) and proliferate when the cooked rice is stored at room temperatures for long time leading to the episodes of intoxication (emetic type) or toxi-infection (diarrhoeal type) due to consumption of such temperature-abused rice. The emetic enterotoxin is selectively produced in rice and vegetable sprouts, milk and milk products, the later mostly being adulterated by the addition of rice powder/flour. The long transportation associated with contineous shaking, of milk from rural areas to urban consumption sites provides an excellent shake culture media for the production of B. cereus emetic enterotoxin.
Table 1 : Zone-wise percent adulteration of different samples of milk with starch
| S. No. Zone No of Samples tested No of positive samples % positive |
| 1. Central 10 2 20 |
| 2. North 10 2 20 |
| 3. East 10 1 10 |
| 4. West 10 1 10 |
| 5. South 10 1 10 |
| 6. Total 50 7 14.00 |
Material and Methods
A total of fifty samples of raw milk (100 ml each) were collected in sterile urocols from local milk vendors of five arbitrary zones of Srinagar city viz. North (Hazratbal and surrounding areas), South (Sonawar and dalgate), East (Harwan, Shalimar and Nishat), West (Qamarwariaandaadjacent areas) and Central (Habbakadal, Khanyar and adjacent areas). The samples (Table 2) were brought to the laboratory on ice and processed within two hours of collection.
Table 2 : Zone wise distribution of Bacillus cereus emetic strains in raw milk
| S. Zone Samples tested Samples contaminated with Percent |
| No B. cereus emetic strains contamination |
| 1. Central 10 3 30 |
| 2. North 10 1 10 |
| 3. East 10 2 20 |
| 4. West 10 1 10 |
| 5. South 10 1 10 |
| 6. Total 50 8 16 |
Detection of starch/ rice flour
The samples of raw milk were subjected to test for the detection of starch/rice flour as an adulterant. For this 1% iodine solution was used as the standard. 3ml of test sample raw milk was thoroughly mixed and boiled for 15min. After cooling to the room temperature 2 to 3 drops of 1 % iodine was added drop by drop. A positive test was indicated by change of colour to blue, which disappeared after boiling and reappeared on cooling.
Table 3: Biochemical and other characteristics of Bacillus cereus emetic strains
| S.No | Biochemical Characteristic |
| 1 | Gram’s reaction |
| 2 | Motility |
| 3 | Lecithino-vitelline reaction |
| 4 | Catalase reaction |
| 5 | Indole production |
| 6 | Nitrate reduction |
| 7 | Starch Hydrolysis |
| 8 | Vogesproskauer test |
| 9 | Fermentation of Ammonium |
| salt sugars viz. | |
| a) Glucose | |
| b) Arabinose | |
| c) Mannitol |
Standard Bacillus cereus emetic strain
The standard Bacillus cereus emetic strain (NCTC 11143) originally recovered from a natural emetic type food poisoning outbreak by Public health laboratory service Coollindale, London was obtained from Department of Veterinary Public Health, Ranchi Veterinary College, Ranchi, Bihar
Table 4
| S.No Biochemical Characteristic ReactionNCTC 11143 Field strains |
| 1 Gram’s reaction + + |
| 2 Motility + + |
| 3 Lecithinovitelline reaction + + |
| 4 Catalase reaction + + |
| 5 Indole production – – |
| 6 Nitrate reduction + + |
| 7 Starch Hydrolysis – – |
| 8 Vogesproskauer test + + |
| 9 Fermentation of sugars viz. |
| a) Glucose + + |
| b) Arabinose – – |
| c) Mannitol – – |
Isolation and Identification
The samples were subjected to the standard procedures for isolation of Bacillus cereus Emetic strains as described by Sakurai et al.(1994) with slight modification.The samples were heated to 70 0C for 10-15 minutes and then cooled down to 25-35 0C for inoculation into brain heart infusion broth (BHIB). The BHIB tubes were then incubated at 370C for 24-48 hours. A loopful of the broth culture was then streaked on subjected to a temperature of 80-90 0C for 2 hours in a preset water bath. The tubes were allowed to cool and subsequently streaked on Mannitol Egg Yolk and polymixin B-sulphate agar plates (MYPA) and incubated at 37 0C for 24 hours. The discrete flat pink colonies with serrated borders, surrounded by a zone of lecithinase reaction and starch negative were taken as Bacillus cereus.The colonies were picked up on nutrient agar slants for biochemical and other tests (Table 3) as confirmatory to B. cereus as described by Cowan (1974),Gibson and Gordan (1974) and Norris et al. (1981). Starch hydrolysis negative colonies were designated as emetic strains of B. cereus.
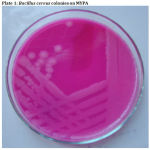 |
Figure 1: Bacillus cereus colonies on MYPA |
Results
Starch Adulteration and Bacillus cereus emetic strain contamination of raw milk
Out of total fifty samples screened, 7 samples were found adulterated with the starch (rice flour) showing an overall prevalence of 14%. All the starch positive samples revealed positive results for Bacillus cereus emetic strain contamination. However one of the starch negative samples also revealed positive results for Bacillus cereus emetic strain contamination making a prevalence of 16%.
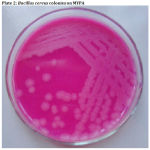 |
Figure 2: Bacillus cereus colonies on MYPA |
Among different representative zones of Srinagar city, central and north zones revealed the highest percentage of starch adulterated milk samples with 2 out of 10 samples (20%) from each zone, followed by west, east and south zones having 1 out of 10 samples positive (10%), as shown in the following table (Table 2).
Milk samples from central zone recorded the highest percentage of Bacillus cereus emetic strains with 3 out of 10 samples positive (30.0%) followed by east zone where 2 out of 10 samples were contaminated. All the other zones i.e. east, west and north zones revealed 10.00 % contamination with Bacillus cereus emetic strains (Table 8).
Cultural and biochemical properties of field isolates of Bacillus cereus emetic strains recovered from milk and milk product
The morphological, cultural and biochemical characteristics of field isolates recovered from raw milk were compared with standard Bacillus cereus strain NCTC 11143. The isolate and the standard strains revealed the similar biochemical tests and morphological characteristics. The results are presented in Table 3 and plates 1 and 2.
Discussion
Raw milk serves as a potential reservoir for many bacterial pathogens. The role of staphylococci, Listeriae and Salmonellae in milk-borne infections and intoxications has been elucidated.
These organisms are easily killed by heat treatment either in the form of pasteurization or boiling, the later being more acceptable method under local conditions. The combined abilities of the spores and toxins, to survive pasteurization and of certain strains to multiply at low temperatures make Bacillus cereus a unique organism as a milk-borne pathogen. There is hardly any organism with such diversified characteristics like aerobic and anaerobic, motile and/or non-motile, psychrotropic (40C) and thermophilic (500C) as Bacillus cereus. These characteristics make Bacillus cereus a unique and one of the most important food poisoning causing organisms. Bacillus cereus is ubiquitous and appears to have adjusted to the environmental changes in such a way that its presence is anticipated in almost all foods of domestic consumption, whether in the form of vegetative cells, spores or preformed toxins, thus posing a great public health threat. Raw market milk, being the source of all the three, acts as a good source of Bacillus cereus food poisoning, either directly or in the form of products utilizing milk as an ingredient. Milk and milk products are naturally contaminated with diarrhoeal strains of Bacillus cereus,which on conventional heating are easily killed as is true with other such food poisoning causing organisms. The presence of emetic strains of B. cereus in milk and of emetic enterotoxins is anticipated in situations where milk is adulterated with starchy foods like rice powder etc.
As milk is transported from the rural production areas to the urban consumer sites, for either direct consumption or as a basic ingredient of other milk products, it acts as an important shake culture medium for toxin production espacially when the ambient temperature is coinciding with the growth requirements of the organism. Besides, absence of cold chain favours the emetic toxin production by the bacteria, even in low numbers. The toxin, because of its heat stability and the capacity to withstand a wider range of pH (2-11), leads to the onset of emetic syndrome in human beings when such contaminated milk is consumed. Milk being utilized as the basic ingredient in many milk products such as cheese, burfi, rasgola, rasmalai and ice-cream, if contaminated with the emetic strains of Bacillus cereus,poses the greatest public health challenge as toxins are not destroyed by any such process employed in the manufacture of these products.
In the present study all the samples of raw milk were subjected to starch test and both starch positive as well as starch negative samples were tested for detection of B. cereus emetic strains by standard procedure. The study revealed 14% adulteration of starch in the samples of the raw milk.
In the present study, all the starch positive samples of the milk revealed presence of Bacillus cereus emetic strains which otherwise form the part of the microflora of the rice and rice products, establishing a fact that rice powder was used as an adulterant for increasing the physical attributes, texture and the bulk without affecting the specific gravity of the milk. However one of the starch negative samples of milk also revealed positive results for Bacillus cereus emetic strains which might have come from water, utensils or any other source such as human handler, dairy environment or dairy animal itself, as reported by other workers (Lin, 1998; Sharma et al., 2003; Abd-El-Rehman. 1988; Anderssonet al.,1995; Rosnneret al., 1990).
Sixteen per cent of market milk samples were contaminated with Bacillus cereus emetic strains. The results are in agreement with the findings of jayachandraet al. (1985) who reported distribution of Bacillus cereus as 12.30 percent in milk samples collected from street vendors, dairy plants, organized dairies and villages, while as Ahmed et al. (1983) reported a prevalence of 21% in raw milk in Egypt. Both these findings are a strong foundation of the fact that the adulteration of market milk with rice flour (powder), to earn more from the less production of milk, is at the cost of human health. Among others Abdel khaliqet al. (1996) found 40.00 and 30.00 percent of raw and pasteurized milk samples, respectively contaminated with Bacillus cereus, while as Sharma et al .(2003) reported 66% contamination of milk with B. cereus.
Contamination of milk by Bacillus cereus has also been reported by many workers and established that entry of Bacillus cereus in the market milk and milk products could be due to many reasons including the adulteration of milk by water and rice flour (Kamatet al., 1989, Lin, 1998), use of contaminated utensils (Giffelet al., 1996) or even the mastitis in the cows (Adlanet al., 1980). Another possible explanation could be the prolonged holding time under unhygienic conditions at retail outlets resulting in higher initial load of Bacillus cereus in raw milk. Recovery of Bacillus cereus from pasteurized milk has also been reported (Wong et al., 1988 and Mudasir, 2009). A higher percentage of contamination in pasteurized (30%) has been reported by Shah et al. (1996).
The presence of Bacillus cereus emetic strains in raw milk is alarming owing to the heat and pH resistance of the bacteria with subsequent production of toxins in milk in stationary phase. So such kinds of adulterations can prove detrimental since Bacillus cereus, besides causing GIT syndromes, is also involved in several non-GIT syndromes like meningitis, endocarditis etc. The present study, therefore unveils the faulty practice of adulteration of milk and milk products with rice powder, which needs a more strong monitoring and frequent testing, followed by subsequent control measures on the part of municipal authorities. Since all the starch positive samples were confirmed for Bacillus cereus emetic strain contamination so the presence of Bacillus cereus may as act as an indicator bacteria for starch adulteration in milk and milk products and vice versa thus advocating total condemnation of starch contaminated milk and milk products, owing to the presence of its toxins.
Although there are only a few reports of food poisoning out breaks due to Bacillus cereus following consumption of milk, yet in most of the cases the organisms were capable of producing emetic enterotoxins (Giffelet al., 1996). The emetic enterotoxin producing strains of Bacillus cereus is of the serious public health significance, since rice powder harbouringBacillus cereus emetic strains will get a suitable media in milk for toxin production, which is not destroyed even at a temperature of 1260C for 90 minutes, causing Bacillus cereus emetic syndrome particularly in children and the older people who are the major consumers of milk. Therefore the mere presence of Bacillus cereus emetic strains in raw milk needs to be reviewed for acceptibility of the later keeping in view the stability of the toxins that are formed. The inherent capacity of these organisms to adapt to a particular food or the presence, in the medium, of substances that promote/suppress enterotoxin production have been proposed (Singh, 1994). This study was primarily initiated to satisfy the need for data, which could be used as the background information in hazard analysis of milk and milk products in different representative zones of Srinagar city. Different zones revealed different percentage of starch and Bacillus cereus emetic strain contamination. In the central zone, a higher contamination percentage of both starch and Bacillus cereus emetic strain in both milk and milk products was observed which could possibly due to increased human activity as a result of higher population density coupled with heavy vehicular stress and poor living conditions.
Although, Bacillus cereus outbreaks were associated mostly with the ingestion of catered meals. Only several outbreaks were mentioned in which milk products could have been the cause of food poisoning. The poisoning, however, seems to be underestimated in the literature and statistics because of its short duration and resemblance to S. aureus food posoning.
However all the isolates revealed similar morphological characteristics and biochemical tests as depicted by the standard strain (NCTC 11143).
References
- Abdel- Khalak, A. and El- Sharbini, M. Prevalence of enterotoxigenicBacillus cereus in raw and pasteurized milk.Vet. Med. J., Giza 44: 157- 161 (1996).(CAB Abstracts, 1996-98/ 07 record 96 of 153).
- Abd-El-Rehman, H. A. and Ahmad, A. A. H. Incidence and level of occurrence of proteolytical microorganisms in some selected foods. Assuit Veterinary Medical Journal, 19 : 72-78 (1988). (CAB abstracts, 1987-1989, Record 2 of 33).
- Adams, M. R. and Moss, M. O., Royal Society of Chemistry Great Britain, England.Food Microbiology.3: 60-64 (2007).
- Adlan, A.M., Shommein, A. M., Amin, E. D. M-El. And El-Amin, E. D. M., A survey of bovine mastitis in four dairy farms in the Sudan.Sudan Journal of Veterinary Research2: 27-39 (1980). (CAB international record 136 of 153).
- Ahmad, A. A. H., Moustafa, M. K. and Marth, E. H., Incidence of Bacillus cereus in milk and milk products. Journal of Food Protection46: 126-28 (1983).
- Andersson, A. U., Granum, P. E. and Ronner, P., What problems does the food industry have with the spore forming pathogens Bacillus cereus and Clostridium perfringens?.International Journal of Food Microbiology28:145-56 (1995).
- Cowan, S. T., Characteristics of gram-positive bacteria. In cown and Steels Manual for the identification of medical bacteria.2ndEdn.Cambridge press, London, Chap. 6: 71 (1974).
- Crerar, S. K., Dalton, C. B., Longbottom, H. M. and Kraa, E., Foodborne diseases: current trends and future surveillance needs in Australia. Medical Journal of Australia165: 672-75.(1996).
- Gibson, T. and Gordan, R. E., Genus 1 :Bacillus in Bergey’s Manual of Determinative Bacteriology. 8th Edn. Eds. Buchanan, N. E. pp.529-550. Baltimore, Williams and Wilkins (1974).
- Giffel-MC-te., Beumer, R. R., Leijendekkers, S., Rombouts, F. M. and TeGiffel, M. C., Incidence of Bacillus cereus and Bacillus subtilisin foods in Netherlands. Food Microbiology 13: 53-58 (1996). (FSTA Current 1990-9/96, Record 12 of 153).
- Granum, P. E., Brynestad, S., O’Sullivan, K. and Nissen, H., Enterotoxin from Bacillus cereus: production and biochemical characterisation. Netherlands Milk Dairy Journal47: 63-70 (1993).
- Hauge, S., Food poisoning caused by aerobic spore forming Bacilli. Journal of Applied Bacteriology18: 591-95 (1955).
- Hussain, S. A., Munshi, Z. H. and Hussain, I., Food poisoning due to Bacillus cereus. A case report.Journal of Veterinary Public Health. 5: 57-59 (2006).
- Jayachandra, T., Krishnamurthi, P. S. and Naterajan, N., Study on the incidence and distribution of Bacillus subtilis and other spore formers in various sources of milk supplies. Cherion, 14 : 98-100 (1985).
- Kamat, P. R., Studies on some properties of emetic and diarrhoeal enterotoxin standard strains of Bacillus cereus. M.V.Sc thesis, submitted to B.A.U., Ranchi, Bihar (1989).
- Kramer, J. M. and Gilbert, R. J., Bacillus cereus and other Bacillus species. M.P Doyle (ed.) Food-borne Bacterial pathogens.pp 21-70 Marcel Dekker, New York (1989).
- Larsen, H. D.andJorgeusen, K, The occurrence of Bacillus cereus in Danish Pasteurised milk, International journal of food microbiology 34: 179-186 (1996).
- Lin, S., Scraft, H., Odumeru, J. A. and Griffths, M. W., Identification of contamination sources of Bacillus cereus in Pasteurized milk. International Journal of Food Microbiology43: 159-71 (1998).
- Lund, T., DeBuyser, M. L. and Granum, P. E., A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Molecular Microbiology.38: 254-61 (2000).
- Mudasir, A. R., Studies on Bacillus cereus in different foods. M.V.Sc. thesis submitted to GADVASU, Ludhiana, Punjab (2009)
- Norris, J. R., Berkeley, R. C. W., Logan, N. A. and O’Donnell, A. G., The genera bacillus and sporolactobacillus. In “The Prokaryotes- A Handbook on Habitats, Isolation and Identification of Bacteria”.Vol. 11. Ed. Starr. M. P., Stolp. H., Truper, H. G., Balows, A. and Schlegal, H. G., pp. 1735 (1981).
- Rosner, U., Husmark, U. and Hendriksson, A., Adhesion of Bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol.69: 550-556 (1990).
- Sakurai, N., Koike, K. A. Y. and Hayashi, H., The rice culture filtrate of Bacillus cereus isolated from emetic type and food poisoning causes mitochondrial swelling in a HEp-2 cell. Microbial.Immunol.38 : 337-343 (1994).
- Schiefer, B., Macdonald, K. R., Klavano, G. G. and Van-Dreumel, A. A., Pathology of Bacillus cereus mastitis in dairy cows. Canadian Veterinary Journal17: 239-43 (1976).
- Schoeni, J. L. and Wong, A. C. L., Bacillus cereus food poisoning and its toxins. Journal of Food Protection68: 636-48 (2005).
- Shah, R. C., Wadher, B. J. and Bhoosreddy, G. L.., Incidence and characteristics of Bacillus cereus isolated from Indian foods. Journal of Food Science and Technology, 33 : 249-250 (1996).
- Sharma, C. S., Sharma, D. K., Gill, J. P. S., Aulakh, R. S. and Sharma, J. K., Bacillus cereus from foods of animal origin in India and its public health significance. ActaveterinariaScandinavica44:118 (2003).
- Singh, D. K. and Narayan, K. G., Effect of hydrogen ion concentration and sucrose on enterotoxin production by Bacillus cereus. Journal of Food Science and Technology, 31: 338-40 (1994).
- Vijaylakshmi, G., Dwarkanath, C. T. and Sreenivasa Murthy, V., Studies on Bacillus cereus contamination of rice and rice preparation. J. Food Sci. Technol. 18: 231-234 (1981).
- Wong, H. C., Chen, Y. L. and Chen, C. L. F., Growth, germination and toxigenic activity of Bacillus cereus in milk products. Journal of Food Protection.51: 707-710 (1988).







