I. Made Sukadana1, Sri Rahayu Santi2 and I. G. K. Arijana2
1Department of Chemistry-Faculty of Science and Maths Udayana University Bali Indonesia.
2Histology Laboratory-Faculty of MedicineUdayana University Bali Indonesia.
Corresponding Author E-mail. im_sukadana@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1291
Abstract
This study aimed to evidence the potent antiatherosclerosis of n-buthanol extract Inocarpus fagiferus Fosb stem bark through biomarker SOD-2 and SOD-3expression aortic endhotelial cell of wistar rats hypercholesterolemia within 4 months of observation. The n-buthanol concentrate extract was applied to wistar rats for 4 months use to the posttest only control group design. Twenty-five wistar rats were randomized into 5 groups such as P0 (negative control), P1 (positive control, hypercholesterolemia), P2 (hypercholesterolemia + n-buthanol extract in the dose of 50 mg/kg bw), P3 (hypercholesterolemia + n-buthanol extract in the dose of 100 mg/kg bw), and P4 (hypercholesterolemia + n-buthanol extract in the dose of 150 mg/kg bw). The study showed that n-buthanol extract stem bark of Inocarpus fagiferus Fosb in the dose of 50 mg/kg bw was able to increase the expression of SOD-2 aortic endhotelial cells in wistar rats hypercholesterolemia significantly (p<0.05), but it was not significantly (p>0.05) for SOD-3 expression.
Keywords
Antiatherosclerosis;Hypercholesterolemia Inocarpus Fagiferus Fosb;SOD-2; SOD-3;
Download this article as:| Copy the following to cite this article: Sukadana I. M, Santi S. R, Arijana I. G. K. The Intake of Inocarpus Fagiferus Fosb Stem Bark N-Buthanol Extract Caused the Increase Expression of Sod-2 and Sod-3 Aortic Endhotelial Cells of Hypercholesterolemia Rats. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Sukadana I. M, Santi S. R, Arijana I. G. K. The Intake of Inocarpus Fagiferus Fosb Stem Bark N-Buthanol Extract Caused the Increase Expression of Sod-2 and Sod-3 Aortic Endhotelial Cells of Hypercholesterolemia Rats. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17704 |
Introduction
Inocarpus fagiferus Fosb etnobotanically is used to prevent ischaemic heart disease and atherosclerosis (Sotheeswaran and Sharif, 1994). As early study that the ethanol extract of gayam stem bark contained total flavonoids and phenols compounds. They are potent to free radicals scavenger DPPH and able to inhibit the formation of lipid peroxide (Santi and Sukadana, 2015). In doses of 50 mg/kg bw, the n-buthanol extract of gayam stem bark were evidenced by increasing the SOD activity, decreasing of the plasm total cholesterol, triglyceride, and malondialdehide levels, but in dose of 100 mg/kg bw it decreasing of the LDL cholesterol and increasing the plasm HDL cholesterol levels in the hypercholesterolemia wistar rats. (Santi et al., 2015). This extract also able to decreases the 8-OHdG level blood serum and expression of ICAM-1 aortic endhotelial cell significantly in doses of 100 mg/kg bw, are biomarker early occured to atherosclerosis (Santi and Sukadana, 2016). Therefore, the n-buthanol extract of gayam stem bark was a source of exogenous antioxidants so that it is expected to trigger SOD’s endogenous antioxidant such as SOD-2 and SOD-3 expression of aortic endothelial cells, are atherosclerotic biomarkers (Fukai et al., 1999; Landmesser et al., 2000; Chan, 2001; Stralin and Marklund, 2001; Fukai et al., 2002; Zelko et al., 2002). Endogenous antioxidants SOD reacts with ROS as a response of the body to prevent the formation of free radical molecules resulting in a decrease in the expression of new SOD (Fukai et al., 2002; Faraci and Didion, 2004). The mechanism is an endogenous antioxidation of SOD by breaking the chain reactions that convert the unstable and reactive superoxide anion into a more stable form of hydrogen peroxide (H2O2), oxygen (O2), and water (H2O).
This study discusses the differences in the expression of SOD-2 and SOD-3 of each treatment group compared with the control group of hypercholesterolemia, as the marker of antioxidant in mitochondria cell and extracellular respectivelly of aortic endothelial cell.
Materials and Methods
Materials and Instruments
The plant material was obtained from a place in Bali. Chemicals and equipment which were used to preparation of n-buthanol extract as early reported (Santi et al., 2015). While, the material that was required to immunohistochemical analysis was aorta endhotel of Wistar rat and chemicals as early reported except primary antibody such as Rabbit Anti-SOD-2 Polyclonal Antibody (Bioss, Cat. bs-1080R) and Rabbit Anti-SOD-3 Polyclonal Antibody (Bioss, Cat. bs-3895R.
Methods
Preparation of n-buthanol Extract of Gayam Stem Bark and its Application on Wistar Rats
Procedure to prepared of n-buthanol extract of gayam stem bark and its application on 5 groups wistar rats as early reported (Santi et al., 2015). The observation was conducted until 16 weeks, after that aorta of all of the rats such as the control groups (P0 and P1) and the treatment groups (P2, P3, and P4) were drawn for expression SOD-2 and SOD-3 analyses. The difference of all variables were analyzed by one way Anova with a = 0.05.
Results and Discussion
Expression of SOD-2 Aortic Endhotelial Cell
Figures 1 describe the average and an overview of positive expression of SOD-2 rat aortic endothelial cells based on immunohistochemical analysis using Rabbit Anti-SOD-2 polyclonal antibody cell marked brown on the edges or the cell nucleus as presented in Figure 2. In normal condition (Po) wistar rats expressed SOD-2 average 7.5 cells, while in hypercholesterolemia condition occured to increasing expression (P1). Stress oxidative condition will trigger to endogenous antioxidant SOD-2 as a resistance effort from radical ions attact, therefore its characteristic subselular of SOD-2 as first defense toward oxidative stress (Packer, 2002). In groups P2 i.e: treatment of hypercholesterolemia added n-butanol extract gayam stem bark in doses of 50 mg/kg bw shown increasing expression significantly toward P1 (p<0.05) which exogenous antioxidants appear to trigger sub-cellular expression of SOD-2 in endothelial cells. The avaerage of expression in groups P3 increase significantly (p<0.05) toward P1 but not with in groups P2 (p>0.05). Although doses in P3 increased but it not effect to expression endogenous antioxidant. The possibility of these was caused by doses of exogenous antioxidant that was given to rat was not enough to catched a free radical attack so that the expression of SOD-2 not significantly. On the other hand in the P4 group, larger doses actually lowered the expression of SOD-2, this is probably due to the reduced amount of free radical ions in the body of the rats so that the endogenous expression of SOD-2 decreases as presented in Figure 1.
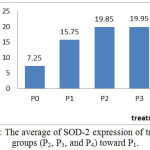 |
Figure 1: The average of SOD-2 expression of treatment groups (P2, P3, and P4) toward P1.
|
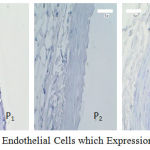 |
Figure 2: Aortic Endothelial Cells which Expression of the SOD-2
|
Expression of SOD-3 Aortic Endhotelial
The average and description of expression SOD-3 positive of rat aortic endothelial cells for control and treatment groups based on immunohistochemical analysis using Rabbit Anti-SOD-3 polyclonal antibodies are presented in Figure 3 and Figure 4. SOD-3 endogenous antioxidants are not expressed in endothelial cells but are extracellularly present and expressed in all blood vessel walls especially between endothelial and vascular muscles (Sandstrom et al., 1992; Stralin et al., 1995; Oury et al., 1996; and Fukai et al., 2002). However, oxidative stress conditions such as in the treatment of P1 endothelial cells still to expressed SOD-3 as a defense against the condition, but the effect of external antioxidant intake as well as in the treatment group P2 and P3 gives increased expression of SOD-3 but not significantly. As shown in Figure 3, the expression of SOD-3 in the P2 and P3 groups did not give significantly difference toward P1 group (p>0.05), even with hypercholesterolemia conditions given exogenous antioxidants in doses of 50 and 100 mg/ kg bw. But in larger doses (P4) of 150 mg/ kg bw it appears that the average SOD-3 expression decreases as well as under normal conditions P0.
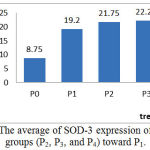 |
Figure 3: The average of SOD-3 expression of treatment groups (P2, P3, and P4) toward P1.
|
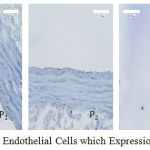 |
Figure 4: Aortic Endothelial Cells which Expression of the SOD-3
|
Antioxidant compounds in n-buthanol extract of gayam stem bark as like flavonoid and phenol can induce endogenous antioxidant SOD-2 in the endothelial cell so that it can expressed high but it can not expressed for SOD-3.
Conclusions
The n-buthanol extract of Inocarpus fagiferus Fosb stem bark potent to prevent atherosclerosis through increasing of SOD-2 expression aortic endothelial cells significantly (p<0.05) in the hypercholesterolemia wistar rats in the doses of 50 mg/kg bw, but it not significantly (p>0.05) for SOD-3 expression.
Acknowledgment
We would like to express our gratitude to RISTEKDIKTI that provided us the fund throughout Hibah Fundamental (2017) and the Rector of Udayana University through the Head of LPPM that facilitated our research.
References
- Chan P.H. Reactive Oxygen Radicals in Signaling and Damage in the Ischemic Brain. J Cerebral Blood Flow Metabol. 2001;21:2-14.
CrossRef - Faraci F.M and Didion S.P. Vascular Protection Superoxide Dismutase Isoforms in the Vessel Wall. Arterioscler Thromb Vasc Biol. 2004;24: 1367-73.
CrossRef - Fukai T, Folz R.J, Landmesser U, Harrison D.G. Extracellular Superoxide Dismustase and Cardiovascular Disease. Cardiovasc Res. 2002;55(2):239–49.
CrossRef - Fukai T, Siegfried M.R, Ushio-Fukai M, Griendling K.K, Harrison G. Modulation of Extracellular Superoxide Dismutase Expression by Angiotensin II and Hypertension. Circ Res. 1999;85:23–8.
CrossRef - Landmesser U, Merten R, Speikermann S, Buttner K, Drexler H, Hornig, B. Vascular Extracellular Superoxide Dismutase Activity in Patients with Coronary Artery Disease. Relation to Endothelium-dependent Vasodilation. Circulation. 2000;101:2264–70.
CrossRef - Oury T.D, Day B.J, Crapo J.D. Extracellular Superoxide Dismutase: a Regulator of Nitric Oxide Bioavailability. Lab Invest. 1996;75:617–36.
- Packer L. Methods in Enzymology. San Diego, Calif, Academic Press. 2002;349.
- Sandstrom J, Carlsson L, Marklund S.L, Edlund T. The Heparin-Binding Domain of Extracellular Superoxide Dismutase C and Formation of Variants with Reduced Heparin Affinity. J. Biol Chem. 1992;267:18205-9.
- Santi S.R dan Sukadana I M. Aktivitas Antioksidan Total Flavonoid dan Fenol Kulit Batang Gayam (Inocarpus fagiferus Fosb). Jurnal Kimia. 2015;9(2):160-168.
- Santi S.R, Sukadana I. M, Siaka I M. The Potential of Total Flavonoids and Phenol Contents in Stem Bark of Gayam (Inocarpus Fagiferus Fosb) as an Antioxidant Through the Decrease of MDA Level, Increase of SOD Activities and Improvement of Lipid Profile in Rats Atherosclerosis Hypercholesterolemia. European Journal of Biomedical and Pharmaceutical Sciences. 2015;2(6):55-58.
- Santi S.R and Sukadana I.M. Antiatherosclerosis n-Buthanol Extract of Inocarpus Fagiferus Fosb Stem Bark Through the Decrease of 8-OHdG Level and Expression ICAM-1 in Rats Hypercholesterolemia. Biomedical and Pharmacology Journal. 2016;9(3):1219-1224.
CrossRef - Sotheeswaran S, Sharif M.R. Lipids from the Seeds of Seven Fijian Plant Species. Food Chemistry. 1994;49:11-3.
CrossRef - Stralin P, Kurlsson K, Johansson B.O, Marklund S.L. The Interstitium of the Human Arterial Wall Contains Very Large Amounts of Extracellular Superoxide. Arterioscler Thromb Vasc Biol. 1995;15:2032-6.
- Stralin P, Marklund S.L. Vasoactive Factors and Growth Factors Alter Vascular Smooth Muscle Cell EC-SOD Expression. Am J Physiol. 2001;281:111621-9.
CrossRef - Zelko I.N, Mariani T.J, Folz R.J. Superoxide Dismutase Multigene Family: A Comparison of the CnZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) Gene Structures, Evolution, and Expression. Free Radical Biol Med. 2002;33:337-49.
CrossRef








