Saidah Fasiha Nordin1,2, Muhammad Luqman Nordin2, Abdinasir Yusuf Osman2, Ruhil Hayati Hamdan3, Rumaizi Shaari2, Mohd Mokhtar Arshad2 and Abd Rahman Aziz3
1Perak State, Department of Veterinary Services, Jalan Sultan Azlan Shah, 30740 Ipoh, Perak, Malaysia.
2Department of Clinical, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa 16100 Kota Bharu, Kelantan, Malaysia.
3Department of Paraclinical, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia.
Corresponding Author E-mail: luqman.n@umk.edu.my
DOI : https://dx.doi.org/10.13005/bpj/1310
Abstract
Human consumption for fish protein has increased but diseases were recognized as the main constraint to the fishery production. Thus, there is the need to enhance feed efficiency, growth performance and disease resistance of cultured aquaculture using safer feed additive such as medicinal herbs. The using of herbs in powder form is not well reported, thus an experiment was conducted to evaluate the best effect of chamomile flowers, Matricaria chamomilla L. concerning their impacts on growth performance, blood profile as well as on the non-specific immune response on Red hybrid Tilapia, Oreochromis sp. fingerlings. A total of 240 fingerlings of Red hybrid Tilapia were used and chamomile powder at the rate of 2%, 4%, 6% were added to the commercial diet. The experimental Tilapia were fed with the four prepared diets including control diet of 0% chamomile powder for 28 days at of 5% of fish biomass. Different growth parameters and blood parameters were assessed to evaluate the growth performance and immune response of the experimented Tilapia. Study revealed that chamomile powder, Matricaria chamomilla L. at the rate of 6% showed the best result in term of growth performance as well as immune response parameters of the experimental Tilapia. Thus, supplementation of chamomile powder, Matricaria chamomilla L. in the rate of 6% as feed additive of fish diet is recommended.
Keywords
Chamomile; Growth Performance; Immune Response; Oreochromis sp; Red Hybrid Tilapia
Download this article as:| Copy the following to cite this article: Nordin S. F, Nordin M. L, Osman A. Y, Hamdan R. H, Shaari R, Arshad M. M, Aziz A. R. The Effect of Matricaria Chamomilla L on the Growth Performance of Red Hybrid Tilapia. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Nordin S. F, Nordin M. L, Osman A. Y, Hamdan R. H, Shaari R, Arshad M. M, Aziz A. R. The Effect of Matricaria Chamomilla L on the Growth Performance of Red Hybrid Tilapia. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17396 |
Introduction
Many millions of people around the globe find a source of income and livelihood in the fisheries and aquaculture sector. With the increasing demand for fish protein, fish or aquaculture industry is also expanding rapidly as Asia became the major player that contributes almost 90% of the world’s aquaculture production.1 Many fish species contain beneficial nutrients such as Vitamin D, Omega-3 and 6, protein and selenium which are important in fetal cognitive development and general body health.2 Therefore, faster in growth performance of capture fish is really important in order to meet the demand and supply.3 Moreover, diseases were also recognized as the main constraint to the development of aquaculture industry globally and resulted in significant growth performance and economic loss to fish farmers.4 Most of the pathogens are opportunistic which infects cultured fish once there is a flaw in the fish’s immediate environment. Bacteria are pathogens that are frequently reported to attack cultured fish followed by viruses, parasites and fungi.1 On the other hand,5 stated that protozoan and bacterial disease were the major diseases associated with fish culture. Therefore, there was the need to improve aquaculture in term of growth and diseases prevention.
Chemicals that include hormones, vitamins, antibiotics among others have long been tested as appetizing, growth promoters, antibacterial and other purposes in mariculture6,7 stated that although these substances have reported to have a positive effect on fishes and shrimps, they cannot be recommended in commercial mariculture operations due to the residual and other undesirable side effects. Antibiotic usage in prophylactic treatment has led to the development of the drug resistant and the need to switch over to other options. The antibiotics also may reduce the larval growth and inhibit defense mechanisms of the fish larvae.7-8 Globally, people have understood concerns over food safety and are largely aware of the adverse effects of these synthetic chemicals which have led to the shift over to organic products.9
Plants have recently been identified as the sources of safer and cheaper alternative options with valuable properties such as no adverse effect on the ecosystem. To date, there is no herbal- resistance nor harmful effect reported in relation to the herbal dietary.10 According to,11 almost all plant species have secondary metabolites known as a phytochemical compound, which responsible not only in plant growth and reproduction but also as defense systems against many pathogens.
Chamomile is one of the most ancient and most widely used medical herbs in the world especially in human medical practices.12 It is a member of the daisy family (Asteraceae or Compositae). The oil has bactericidal and fungicidal activities, particularly against Staphylococcus aureus and Candida albicans. On the other hand, the dried flowers of chamomile contain many terpenoids and flavonoids which are vital to its medicinal properties.12 It was also reported that dried chamomile flowers at the rate of 2% increased all growth parameters (body weight, weight gain, and specific growth rate) in Tilapia fingerlings (Oreochromis niloticus) and significantly reduced the Feed Conversion Ratio (FCR) and increased survival rates.13
The usage of feed additives can improve animal health and performance.14 However, the usage of chemicals such as the antibiotics had led to resistant strains, reduce larval growth and inhibit defense mechanisms of fish larvae among other.15 Thus, medicinal plants have been alternatively suggested to improve nutrient utilization and absorption or stimulation of the immune system.16 Therefore, the aim of this study is to evaluate the effect of chamomile flowers concerning their impacts on growth performance and blood profile as well as on the non-specific immune response of the tested fish.
Materials and Method
Experimental Design
A total of 240 of Red hybrid Tilapia fingerlings with average body weight of 20 gram and total length of 4 inches were used in this study. The fish was purchased from the local farmer in Kelantan bred in aquaria and transferred to the facilities of Universiti Malaysia Kelantan at the beginning of the experiments. All the red Tilapia fingerlings were randomly assigned into four major groups that fed with different concentrations of chamomile powder rate as given in figure 1.
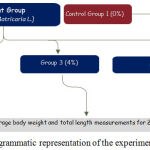 |
Figure 1: Diagrammatic representation of the experimental design.
|
Plant Material and Extract Preparation
The aerial parts of Chamomile (Matricaria chamomilla L.) were collected during the flowering and vegetative phase of the plant at different localities characterized by diverse geographic and climate conditions in Malaysia. The plants were cut at ground level and those portions from above ground were air dried for two weeks in a laboratory of the Faculty of Veterinary Medicine, Universiti Malaysia Kelantan. The chopped, air dried plant material was stored in refrigerator, and waited for extraction analysis. Thereafter, dried plants of Chamomile (Matricaria chamomilla L.) were grounded into powder.
Four experimental diets were formulated from fish commercial diet. A powdered feed (40% protein) was processed to form balls after mixing with different composition of the chamomile (0%, 2%, 4%, and 6% of feed). The fish were fed at a feeding rate of 5% of fish biomass. A little amount of water was added to the mixtures to make it pasty and keep in the freezer in the form of balls and fed to the fish in smaller form to avoid losses in water. The Red hybrid Tilapia fingerlings were fed twice a day (morning and evening) on rations with four different concentrations for 28 days of experimental period. Table 1 shows the distribution of fish treated with Chamomile powder. All experimental fish from each aquarium were sampled and evaluated at the end of the experiment for final average body weight and total length measurements.
Table 1: Distribution of fish before and throughout treatment
| Group | Number (n) | Chamomile powder rate feed (%) | |
| 1 | 60 | 0 | |
| 2 | 60 | 2 | |
| 3 | 60 | 4 | |
| 4 | 60 | 6 |
Evaluation of growth parameters
Different growth parameters that include net weight gain, daily weight gain, specific growth rate (SGR), feed conversion ratio (FCR), and condition factor (K) were calculated using the following equations:-
Weight gain (WG) = Final weight – Initial weight
![]()
Where:
Ln =the natural logarithm,
Wα = Final weight at certain period (g),
Wβ = Initial weight at the same period (g),
t = Period (d).17
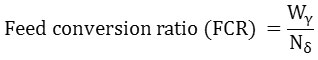
Wγ = Weight of food supplied to the fish during the study period
Nδ = Net fish period18
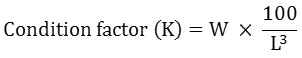
Where:
W = fish weight (grams),
L = fish length (cm). It represents the relationship between lean body weight and body length of the fish.19
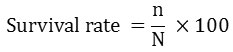
Where
n = number of surviving fish after 28 days
N = number of surviving fish after 28 days20
Evaluation of Immune Response
Blood Parameters
Blood samples (at least 0.5 ml) were collected from the caudal vein of the fish and stored in an EDTA tube for blood analysis. Haemoglobin (Hb), haematocrit (PCV), total red blood cell, white blood cell and differential leucocyte counts were analyzed.
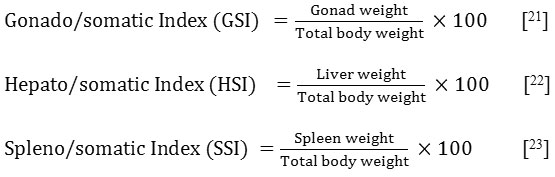
Gonado/somatic, Hepato/somatic and Spleno/somatic Indices: The average of those indices was calculated according to the formulas below:
Bacterial Challenge Test
Bacterial challenge test was conducted using a virulent strain of Streptococcus agalactiae. Ten fishes per aquaria were injected with intraperitoneally (I/P) 0.5mL of 0.5×106 cfu/ml 24 of 24 hour Tryptic Soy Agar culture of S. agalactiae. The fish were kept for 2 weeks during which clinical signs and daily mortality were recorded.
The cumulative mortality percentage (%) and relative percent survival were calculated according to the formulas below:
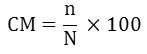
Where
CM = Cumulative mortality
N = Total of dead fish for each treatment
N = Initial total number of fish
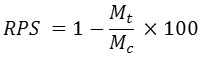
Where
RPS= Relative Percent Survival
Mt = Treatment Mortality
MC = Control mortality 25
Statistical Analysis
All data were analyzed statistically as well as biologically. Evaluation of the effects was performed using oneway Anova and one sample t test with significance level of 0.05 (α=0.05).
Results
Evaluation of Growth Parameters of Treated Tilapia Fingerlings
Table 2, Figure 2, 3, 4, 5 and 6 showed total biomass, average body weight, average body length, mortality and survival rate of Tilapia fed with chamomile for 28 days, respectively. There is significant different of total biomass between groups treated with chamomile powder in the rate of 0% (treatment 1/ control group), 2% (treatment 2), 4% (treatment 3), and 6% (treatment 4) whereby Tilapia in treatment 6% had the highest total biomass while fish in treatment 2% had the lowest total biomass in this experiment. There are no significant changes of average body weight among all groups.
Results on Table 3 and Figure 7, 8, 9, 10 and 11 revealed the changes of net and daily weight gain, specific growth rate (SGR), condition factor, and feed conversion ratio of Tilapia fed with chamomile between the groups for 28 days after treatment with chamomile powder, respectively. The Tilapia in treatment group of 6% chamomile powder showed the highest estimates of net and daily weight gains as well as SGR. On the other hand, fish in both treatment groups (2% and 6%) showed the lowest FCR. Thus, both treatment groups insignificantly had the best FCR compared with the fish of the control group.
Table 2: Total biomass, average body weight, average total length, mortality and survival rate of Tilapia fed with chamomile for 28 days
| Parameters | Serial no. of aquaria (replicates) | Treatment (%) | |||
| 0% | 2% | 4% | 6% | ||
| No. of fish | 1 | 18 | 15 | 19 | 17 |
| 2 | 14 | 19 | 18 | 20 | |
| 3 | 18 | 19 | 19 | 19 | |
| Mean | – | 16.7±1.33 | 17.71.33 | 18.70.33 | 18.70.88b |
| Total biomass (g) | 1 | 250 | 225 | 240 | 258 |
| 2 | 252 | 200 | 245 | 260 | |
| 3 | 252 | 225 | 230 | 285 | |
| Mean | – | 251.30.66b | 216.78.33ad | 238.34.41d | 267.78.68b |
| Total body weight (g) | 1 | 13.9 | 15.0 | 12.6 | 15.2 |
| 2 | 18.0 | 10.5 | 13.6 | 13.0 | |
| 3 | 14.0 | 11.8 | 12.1 | 15.0 | |
| Mean | – | 15.31.35 | 12.51.32 | 12.80.44 | 14.40.70 |
| Total body length (inch) | 1 | 42.1 | 42 | 39.7 | 40.3 |
| 2 | 41.8 | 41.1 | 39.7 | 41.3 | |
| 3 | 43.3 | 42.8 | 41.4 | 40.8 | |
| Mean | – | 42.40.45c | 42.00.491 | 40.20.56a | 40.80.27 |
| Mortality | 1 | 2 | 5 | 1 | 3 |
| 2 | 6 | 1 | 2 | 0 | |
| 3 | 2 | 1 | 1 | 1 | |
| Mean | – | 3.31.33 | 2.31.33 | 1.30.33 | 1.30.88 |
| Survival rate (%) | 1 | 90 | 75 | 95 | 85 |
| 2 | 70 | 95 | 90 | 100 | |
| 3 | 90 | 95 | 95 | 95 | |
| Mean | – | 83.36.66 | 88.36.667 | 93.31.66 | 93.34.41 |
aSignificant different versus control group (p<0.05)
bSignificant different versus 2% group (p<0.05)
cSignificant different versus group (p<0.05)
Table 3: Initial, final body weight, net weight gain, daily gain, relative growth rate, condition factor, and feed conversion ratio of Tilapia fed with chamomile for 28 days
| Parameters | Serial no. of aquaria (replicates) | Treatment (%) | |||
| 0% | 2% | 4% | 6% | ||
| Initial body weight (g) | 1 | 12.0 | 13.7 | 11.0 | 10.0 |
| 2 | 12.5 | 11.0 | 12.0 | 10.0 | |
| 3 | 12.5 | 11.4 | 10.8 | 12.5 | |
| Mean | – | 12.30.16 | 12.00.83 | 11.30.38 | 10.80.83 |
| Final body weight (g) | 1 | 13.9 | 15.0 | 12.6 | 15.2 |
| 2 | 18.0 | 10.5 | 13.6 | 13.0 | |
| 3 | 14.0 | 11.8 | 12.1 | 15.0 | |
| Mean | – | 15.31.35 | 12.51.32 | 12.80.44 | 14.40.69 |
| Net weight gain (g) | 1 | 1.9 | 1.4 | 1.6 | 5.2 |
| 2 | 5.5 | -0.5 | 1.6 | 3.0 | |
| 3 | 1.5 | 0.5 | 1.4 | 2.5 | |
| Mean | – | 3.01.27 | 0.50.52d | 1.50.08 | 3.60.82b |
| Daily weight gain (g) | 1 | 0.067 | 0.048 | 0.058 | 0.185 |
| 2 | 0.196 | -0.017 | 0.058 | 0.107 | |
| 3 | 0.054 | 0.018 | 0.048 | 0.089 | |
| Mean | – | 0.1060.04 | 0.0160.0d | 0.0550.00 | 0.1270.02b |
| Specific growth rate (SGR) | 1 | 0.5 | 0.3 | 0.5 | 1.5 |
| 2 | 1.3 | -0.2 | 0.4 | 0.9 | |
| 3 | 0.4 | 0.2 | 0.4 | 0.7 | |
| Mean | – | 0.70.28 | 0.10.14d | 0.50.02 | 1.00.24b |
| Condition factor (K) | 1 | 0.001 | 0.001 | 0.001 | 0.001 |
| 2 | 0.002 | 0.001 | 0.001 | 0.001 | |
| 3 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Mean | – | 0.0010.00 | 0.0010.00 | 0.0010.00 | 0.0010.00 |
| Feed Conversion Ratio (FCR) | 1 | 9.0 | 12.6 | 10.4 | 3.3 |
| 2 | 3.1 | -35.9 | 10.6 | 5.7 | |
| 3 | 11.3 | 34.5 | 12.5 | 6.8 | |
| Mean | – | 7.82.45 | 3.72.08 | 11.20.68 | 5.31.03 |
bSignificant different versus 2% group (p<0.05)
dSignificant different versus 6% group (p<0.05)
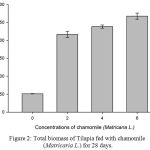 |
Figure 2: Total biomass of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
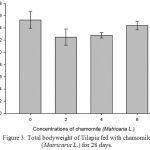 |
Figure 3: Total bodyweight of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
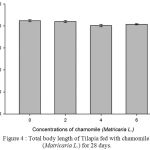 |
Figure 4: Total body length of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
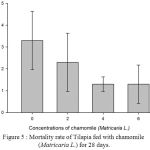 |
Figure 5 : Mortality rate of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
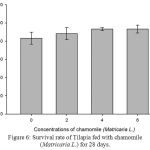 |
Figure 6: Survival rate of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
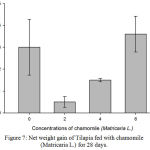 |
Figure 7: Net weight gain of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
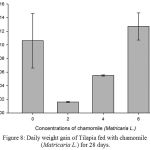 |
Figure 8: Daily weight gain of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
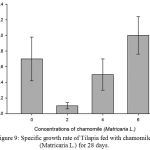 |
Figure 9: Specific growth rate of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
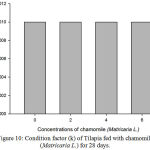 |
Figure 10: Condition factor (k) of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
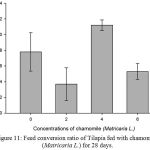 |
Figure 11: Feed conversion ratio of Tilapia fed with chamomile (Matricaria L.) for 28 days.
|
Evaluation of Immune Response of Tilapia Fingerlings
Survival Rate
As in Table 2 and Figure 6, there is no significant difference in the survival rate within all treatment groups including the control group. However, all treatments showed increased survival rate as compared with the control group while fish in the treatment 3 and 4 (chamomile powder in the rate of 4% and 6%) showed the highest survival rate among the groups.
Blood Parameters
From Table 4 shows Tilapia treated with chamomile at the rate of 2%, 4%, and 6% showed significantly lower total leucocytic count compared to control group. Fish in treatment 2 and 3 (chamomile powder in the rate of 2% and 4%) revealed significantly reduced haemoglobin and hematocrit values compared to control group. However, Tilapia in 6% treatment group (treatment 4) showed significantly higher in haemoglobin and hematocrit as compared with fish in the control group.
Gonado/Somatic, Spleno/Somatic and Hepato/Somatic Indices
From Table 5 shows fish in all treatment groups of chamomile powder (chamomile powder in the rate 2%, 4% and 6%) had significantly reduced gonado/somatic and hepato/somatic indices compared with that of fishes in control group. The spleno/somatic index however revealed a significant increase in treated groups compared to the control group.
Challenge Results
Neither significant mortalities nor abnormal clinical signs were noticed among the injected Tilapia in all treatment groups.
Table 4: Total and differential leucocytic counts, haemoglobin content and hematocrit value of Tilapia fed with chamomile for 28 days
| Differential Leucocytic count | Total Leucocytic count | Average Total Leucocytic count | Haemoglobin (Hb) | Hematocrit | |||
| T (%) | Granulocytes | Monocytes | Lymphocytes | ||||
| 0 | 2.5 | 6.4 | 104.8 | 113.7 | 37.9 | 3.7 | 13.1 |
| 2 | 1.8 | 5.2 | 98.5 | 105.5 | 35.167 | 2.9 | 9.0 |
| 4 | 0.5 | 0.5 | 46.9 | 47.9 | 15.967 | 1.3 | 3.3 |
| 6 | 61.4 | 5.7 | 5.4 | 72.5 | 24.167 | 9.4 | 16.2 |
Table 5: Total body, gonads, spleen, and liver weight as well as gonado/somatic, spleno/somatic and hepato/somatic indices of Tilapia fed with chamomile for 28 days
| T (%) | Total body weight (g) | Gonads weight (g) | Spleen weight (g) | Liver weight (g) | Gonado/ somatic index | Spleno/somatic index | Hepato/somatic index |
| 0 | 15.296 | 0.082 | 0.089 | 0.662 | 0.533 | 0.582 | 4.328 |
| 2 | 12.456 | 0.036 | 0.082 | 0.4855 | 0.285 | 0.654 | 3.898 |
| 4 | 12.783 | 0.047 | 0.099 | 0.5395 | 0.368 | 0.774 | 4.221 |
| 6 | 14.392 | 0.035 | 0.122 | 0.5705 | 0.240 | 0.848 | 3.964 |
Note: T = Treatment
Discussion
The available recommendation from previous studies have tested chamomile extract26 and powder.27 Both found that chamomile Matricaria chamomilla L. in the rate of 1% powder and 5% extract showed the best estimates of growth parameters, blood parameters and protection against Aeromonas hydrophila bacterial infection challenge. The rate of 2% and 4% were not studied before while the extract will be very expensive and will add a lot of additional cost to the fish feed. One kilogram of chamomile may produce only 10g of extract; hence chamomile powder was tested in a trial to reduce the cost of feeding the fish of farmer in Malaysia and make it feasible and economic to aquaculture industry
The treatments at the rate of 4% and 6% of chamomile powder were able to increase the total biomass in the treated Tilapia. However, fish in treatment 2 (chamomile powder in the rate of 2%) had the lowest biomass and average body weight in this experiment. This could be attributed to the insignificant high mortality in the group. This also could account for the higher but insignificant average body weight gained by the control group as less fish received the same amount of food compared with other treatment which with a higher number of fish. Meanwhile, Tilapia in treatment 4 (chamomile powder in the rate of 6%) showed the highest estimates of net and daily weight gains as well as SGR. The fish of the both groups (treatment 2 and 4) also show the lowest FCR compared to other groups of treatment including the control group. Therefore, this is in agreement with previous study by28 which stated that dried chamomile flowers at the rate of 2% increased all growth parameters (BW, WG, and SGR) of the experimental Tilapia fingerlings, Oreochromis niloticus and significantly increased FCR and survival rate. This present study also supports by29 finding which recorded that addition of chamomile in fish diets leads to increase of feed intake, protein and energy efficiency ratios along with obvious improvement in FCR. In this trial, the positive effect of chamomile powder treatment on growth performances especially at high level (6g/100g diet) may be due to the increase in protein utilization for the chamomile powder and its pharmaceutical properties.29 This is also supported by30 who stated that some phytobiotics (thyme (Thymus vulgaris), fenugreek (Trigonela foenum graecum) and neem (Azadirachta indica), included in aquatic organisms’ diets, may lead to an increase of productivity and efficiency of feed utilization, at Tilapia species.
The insignificant highest survival rate of fish at treatment rate of 6% chamomile powder compared with that of fish in the control group could be associated with the immune stimulation effect induced by chamomile on the non-specific immune response of Tilapia under study. This result was supported by study under27 which stated chamomile flowers may induce non-specific immune response of the catfish. Experimental Tilapia in all treatments of chamomile rate had low total leucocytic count compared to control group. This result thus disagreed with those recorded by,27 but concordance with those reported by31 who found no significant effects on total leucocytic count. Meanwhile, fish treated with chamomile at the rate of 6% showed the highest figures of haemoglobin and haematocrit values. These results are in agreement with the results of32 and also supported by study conducted by33 that suggested that the more active fishes tend to have higher haemoglobin values than the sedentary ones. The high total leucocytic count of the control group may be due to protein utilization. This supported by34 which stated that fish fed a diet containing high protein have higher macrophage migration compared to fish fed diets with lower protein content. The control group also may have higher severity of stress compared to other treatment groups. Changes in experimental Cyprinus carpio leucocyte system was manifest in the form of leucocytosis with heterpohilia and lymphopenia which are characteristic leucocytic response in animals exhibiting stress.35 Fish in all treatments of chamomile powder (treatment at the rate of 2%, 4% and 6%) had significantly reduced gonado/somatic and hepato/somatic indices when compared with that of fish in control group. The spleno/somatic index however revealed significantly increase in figure versus the control group. Thus, chamomile flower enhanced the development of spleen, the main blood forming organs in fish (in addition to the anterior kidney and thymus) and as a consequence, stimulated the immune response of Tilapia. There were neither significant mortalities nor abnormal clinical signs noticed among the Tilapia in all treatments injected with S. agalactiae. This could be attributed to the high natural or genetic tolerance of the red tilapia, Oreochromis sp. towards S. agalactiae. This similar to,36 who suggested that the MCP-8 gene play an important role in the resistance to S. agalactiae in Tilapia. In agreement by,37 bacterial strain or virulence, bacterial concentration, fish species, water temperature, water quality, and stocking density are factors that can influence the mortality rate and onset of clinical signs under experimental conditions. LD50 of red Tilapia was 3.0×1010 cfu/ml.38-39 The absence of clinical signs during the experiment may be also contributed by an effective cellular immune response established by the fish’s immune system. The previous study also observed that 50% mortality was obtained at the higher bacterial concentrations (9×107 and 9×108 cfu/ml) in experimental Tilapia, Oreochromis nilotus.38
Conclusion
This study investigated the effect of chamomile powder, Matricaria chamomilla L. on Red hybrid Tilapia for their growth and health performances. The present study was suggested that the growth performances and immune response of Tilapia fish in treatment rate of 6% chamomile powder had the best figures rather than fish in other treatments (2% and 4%) including the control group. In addition, the fish were observed to be more active in the treated aquaria. The development of the experimental S. agalactiae infection in the Tilapia could be influence by bacterial strain or virulence of the isolate, bacterial concentration, fish species, and the environment in the challenge system. Varieties of factors have to be manipulated to establish a reproducible experimental challenge that produces similar disease characteristics to the natural infection in fish.38 Therefore, the supplementation of chamomile, Matricaria chamomilla L. in the rate of 6% to the artificial or commercial feeds as immune-stimulating additive can improve the growth performance of Red hybrid Tilapia as well as their immune status.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Acknowledgement
This article is self-funding.
References
- Lee S.W and Wendy W. Diseases in Aquaculture. Research Journal of Animal and Veterinary Sciences. 2014;7(1):1-6.
- McGuire J, Kaplan J, Lapolla J and Kleiner R. The 2014 FDA assessment of commercial fish: practical considerations for improved dietary guidance. Nutrition Journal. 2016;15(1):66.
CrossRef - Juergen V. Prospects for Fisheries and Aquaculture. 2015.
- FAO, Food and Agriculture Organization of the United Nations. 2007 FAO yearbook. Fishery and aquaculture statistics. http://www.fao.org/fishery/publications/ yearbooks/en. 2009.
- Sayuthi S. Fish Diseases in Malaysia: Status and Problems. Proceedings of the Aquaculture Workshop for SEAFDEC/AQD Training Alumni, 8-11 September 1992, Iloilo, Philippines. 1993;57-61.
- Jayaprakas V, Sambhu C. Growth response of white prawn, Penaeus indicus to dietary L-carnitine. Asian Fish Sci. 1996;9:209-219.
- Citarasu T. Herbal Biomedicines: A New Opportunity for Aquaculture Industry. Aquacult Int. 2009;18:403-414.
CrossRef - Atal C.K. Chemistry of some biologically active Indian medicinal plants. Proceedings of the Indian National Science Academy. Part A. Physical sciences. 1982.
- Al-Musalam L.S, Al-Ameeri A. A, Saheb A.S, Al-Yaqout A. Effect of Herbal Feed Additive on the Growth, Survival and Immune Response of Green Tiger Prawn (Penaeus semisulcatus). Pakistan Journal of Nutrition. 2014;13(7):366-371.
CrossRef - Ramudu K.R, Dash G. A Review on Herbal Drugs against Harmful Pathogens in Aquaculture. American Journal of Drug Discovery and Development. 2013;3:209-219.
CrossRef - Nordin M. L, Kadir A.A, Zakaria Z.A, Othman F, Abdullah R and Abdullah M. N. H. (2017). Cytotoxicity and Apoptosis Induction of Ardisia crispa and Its Solvent Partitions against Mus musculus Mammary Carcinoma Cell Line (4T1). Evidence-Based Complementary and Alternative Medicine. 2017.
CrossRef - Srivastava J.K, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. NIH Public Access. 2010.
- Baker M.N and Mostafa M. A. A. The importance of Betafin or chamomile flower as natural feed additives for Nile tilapia fry cultured under cold season conditions. 2006.
- Mansoura Univ. J. Agric. Sci. 31(7):4145-4153.
- Blezinger S.B. (2002). Feed Additive Can Improve Performance of Herd. Available at: http://www.cattletoday.com/archive/2002/November/CT242.shtml. Accessed.May 11 2015.
- Brown J.H. Antibiotics: their use and abuse in aquaculture. World Aquacult. 1989;20(2):34-43.
- Kumar M, Kumar V,Roy D, Kushwaha R and Vaiswani S.Application of herbal feed additives in animal nutrition-a review.Int .J. Lives Res. 2014;4:1-8.
CrossRef - Lugert V, Thaller G, Tetens J, Schulz C & Krieter J. A review on fish growth calculation: multiple functions in fish production and their specific application. Reviews in Aquaculture. 2014.
- Sahzadi T, Salim M & Um-e-kalsoom K. S. Growth performance and feed conversion ratio (FCR) of hybrid fingerlings (Catla catla X Labeo rohita) fed on cottonseed meal, sunflower meal and bone meal. 2006.
- Htun‐Han M. The reproductive biology of the dab Limanda limanda (L.) in the North Sea: gonosomatic index, hepatosomatic index and condition factor.Journal of Fish Biology. 1978;13(3):369-378.
CrossRef - Imgbian T.D and Zarmai T. I. Growth Performance and Survival of Heterobranchus longifilis Fingerlings Fed Graded Levels of Palm Oil Sludge. PAT. 2011;7(1):55-63.
- King M. Fisheries Biology Assesment and Management. Fishing News Books Blackwell Science Ltd., London. 1995;341.
- Yun B, Ali Q, Mai K, Xu W, Qai G and Luo Y. Synergistic effects of dietary cholesterol and taurine on growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets. Aquaculture. 2012;(324-325):85-91.
CrossRef - Dekić R, Savić N, Manojlović M, Golub D & Pavličević J.Condition factor and organosomatic indices of rainbow trout (Onchorhynchus mykiss, Wal.) from different brood stock. Biotechnology in Animal Husbandry. 2016;32(2):229-237.
CrossRef - Bullock G.L. Streptococcal Infections of Fishes. US Fish & Wildlife Publications. Paper 127.1981.
- Maqsood S, Singh P, Samoon M.H & Balange A.K. Effect of dietary chitosan on non-specific immune response and growth of Cyprinus carpio challenged with Aeromonas hydrophila. International Aquatic Research. 2010;2(2):77-85.
- Khairie I, Safuan M, Abdelhadi Y. M. “Chamomile; Matricaria chamomilla; The magic herb in aquaculture”. World Aquaculture Society. 2012.
- Abdelhadi Y.M, Salleh O. A, Sakr S. F. Study on the Effect of Wormseed Plant, Artemisia cina L. and Chamomile, Matricaria chamomilla L. on Growth Parameters and Immune Response of African Catfish, Clarias gariepinus. Journal of Fisheries International. 2010;5(1):1-7.
CrossRef - Baker M.N, Mostafa M. A. A. The importance of Betafin or chamomile flower as natural feed additives for Nile tilapia fry cultured under cold season conditions. Mansoura Univ. J. Agric. Sci. 2006;31(7):4145- 4153.
- Abdel- Tawab A.M. Evaluation of Using Garlic and Chamomile Oils as Feed Additives in Diets of Nile tilapia, Oreochromis niloticus Fry. African J. Biol. Sci. 2010;6(1):73-82.
- Alina A, Cristea V, Dediu L, Grecu I, Docan A, Vasilean I, Mocanu M.C, Petrea S.M. The Influence of Some Phytobiotics on Growth Performance at Oreochromis Niloticus Reared in an Intensive Recirculating Aquaculture System. University of Agricultural Sciences and Veterinary Medicine lasi. 2013;60.
- Adelhamid A. M, Mehrim A. I, El- Barbary M. I, Ibrahim S.M, Abd El- Wahab A.I. Evaluation of a New Egyptian Probiotic by African Catfish Fingerlings. Journal of Environmental Science and Technology. 2009;2(3):133-145.
CrossRef - Hayam D.T, Samy H.M, Eman H. L, Mohamed A. Z. Effect of some medicinal plants diets on the mono-sex Nile Tilapia (Oreochromis niloticus), growth performance, feed utilization and some physiological parameters. Egypt J. Aquat. Biol. & Fish. 2011;15(2):53-72.
- Bhilave M.P, Nadaf S. B, Nalawade V. B, Bhosale S.V. Hematological Profile of Labeo Rohita Fed on Formulated Feed. Bionano Frontier.2014;2:273-277.
- Lee C.S, Lim C, Gatlin III D.M, Webster C. D. Dietary Nutrients, Additive, and Fish Health, Wiley Blackwell. 2015;29–31.
CrossRef - Anonymous. Effect of stress factors on the survival and immune response of Cyprinus carpio against A. hydrophila infection. Department of Marine Biology, Microbiology and Biochemistry, School of Marine Science, CUSAT. Available. 2010. at: http://shodhganga.inflibnet.ac.in/bitstream/10603/39606/12/12_chapter%206.pdf. Accessed June 9 2015.
- Fu G. H, Wan Z. Y, Xia J. H, Liu F, Liu X. J, Yue G.H. The MCP-8 gene and its possible association with resistance to Streptococcus agalactiae in tilapia. Fish Shellfish Immunol. 2014;40(1):331-336.
CrossRef - Wongsathein D. Factors Afecting Experimental Streptococcus Agalactiae Infection in Tilapia, Oreochromis Niloticus. Institute of Aquaculture,University of Stirling. 2012;49-72.
- Amal A. M.N, Nur-Nazifah M, Siti-Zahrah A, Sabri M.Y, Zamri-Saad M. Determination of LD50 for Streptococcus Agalactiae Infections in Red Tilapia and GIFT. 8th International Symposium on Tilapia in Aquaculture. 2008;1245-1251.








