Manuscript accepted on :October 04, 2017
Published online on: --
Department of Molecular and Medical Biotechnology College of Biotechnology, Al-Nahrin University, Baghdad, Iraq.
Corresponding Author E-mail: dr.mahahameed@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1311
Abstract
Nephrotic syndrome is characterized by increased glomerular permeability leading to huge proteinuria, a clinical manifestation found in nephrotic syndrome i.e., hyperlipidemia. Total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) were determined in a study of 42 Iraqi children, half of which were nephrotic and other half healthy individuals as control group. TC, TG, and VLDL of nephrotic group was significantly higher (P<0.05) compared to control group, while HDL of both the groups remained almost the same (P>0.05). LDL of nephrotics was significantly lower (P<0.05) than healthy individuals.
Keywords
Hyperlipidemia; Lipid Metabolism; Nephrotic Syndrome and Total Cholesterol
Download this article as:| Copy the following to cite this article: Al-Bahrani M. H. A. The Evolution of lipid Metabolism in Iraqi Children with Nephrotic Syndrome. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Al-Bahrani M. H. A. The Evolution of lipid Metabolism in Iraqi Children with Nephrotic Syndrome. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17259 |
Introduction
Nephrotic syndrome (NS) is the most frequent glomerular diseases that affect mainly children more strictly, NS is defined as massive proteinuria, hypoalbuminemia, edema, and hyperlipidemia. The proteinuria in childhood nephrotic syndrome is relatively selective and primarily composed of albumin [1]. The decline in albumin level during NS is due to damaged glomerular filtration membrane in the renal cortex. Most patients with renal syndrome are suspected to relapse without complications. However, a few of these NS patients develop certain lesions of focal segmental glomerulosclerosis and suffer from severe but prolonged proteinuria, facing higher risks for complications [2]. Some epidemiological evidences stated a higher incidence of NS in children in Asia compared to Western world [3]. Over 90% of NS children have idiopathic NS that directly linked with primary glomerular disease [4]. Albumin is the major proteins of human plasma (60%) that are being produced from liver with traces also been found in. the extracellular space [5]. In NS the metabolism of lipid and lipoproteins may aid in the progression of various other diseases such as cardiovascular and kidney disorders. In addition, The disorders of lipid metabolism in nephrotic syndrome lead to reduce delivery of lipid fuel to the muscles for energy storage and that contribute to the reduction of body mass and impire exercise capacity. [6]. The current study will focus on the effect of NS on lipoproteins metabolism to find the lipid levels in children. Along with this nephrotics to best help determine a risk factor for developing heart disease and other systematic disorders.
Pathophysiology
Normally, in a healthy individual less than 50 mg/day of urine albumin may be re-absorbed by the tubules[7].while amounts of urinary albumin over 500 mg/day refer to glomerular disease. Various studies involving animal models proposed that huge amounts of albumin (≈80 mg) passage into the urine each day along with equivalent substantial tubular uptake of albumin [8]. However, in human volunteers tubular transport defects suggested that the glomerular urinary albumin concentration is 3.5 mg/L The glomerular capillaries are internally lined with fenestrated endothelium placed on the glomerular basement membrane, which is further covered by glomerular epithelium or podocytes by wrapping the capillaries with cellular extensions called foot processes and filtration slits. These three structures —the fenestrated endothelium, glomerular basement membrane and glomerular epithelium are the main glomerular filtration barrier [9]. which is further explained schematically (Figure 1).
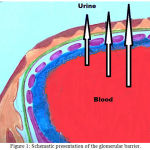 |
Figure 1: Schematic presentation of the glomerular barrier.
|
Filtration of plasma water and solutes is extracellular function of the body, taking place through the endothelial fenestrae and filtration slits. The importance of the podocytes and filtration slits are shown in genetic diseases. In congenital NS of the Finnish population, the gene for nephrin encoding a protein of the filtration slit is being mutated and leading to NS in early childhood. Podocin, a protein being expressed by the podocytes became abnormal in a number of children with steroid-resistant focal glomerulosclerosis.
The changes in glomerular structures may cause proteinuria, mainly due to damage in the endothelial surface, the glomerular basement membrane and the podocytes. Different mechanisms have been seen in different types of NS such as albuminuria alone may occur or in severe cases leakage of all plasma proteins (ie, proteinuria) may take place. Proteinuria that is more than 85% albumin is selective proteinuria. Nonselective proteinuria, being a glomerular leakage of all plasma proteins is not involve in changing glomerular net charge but rather a generalized defect in permeability. This scheme does not permit clear-cut separation of causes of proteinuria, except in minimal-change nephropathy where proteinuria is quiet selective [10].
Pathogenesis
NS is caused by various disorders that damage the clusters glomeruli of the kidney and includes:
Primary causes of NS Abnormal kidney function is a most frequent cause of NS in children, in which kidney tissue appears normal or nearly normal under microscope.
Focal segmental glomerul sclerosis. this condition can characterized by scattered scarring in the glomerulus, this may result from another disease or a genetic disorder
Membranous nephropathy, which is characterized by thickening of the membranes within the glomeruli, supposed to be associated with other diseases including hepatitis B, malaria and lupus [11],[12].
Hereditary nephropathies: These include congenital and hereditary focal glomerulosclerosis resulted from gene mutations coding for podocyte proteins e.g. nephrin, podocin, or the cation channel 6 protein [13].
Common secondary causes include the various systemic diseases such as diabetes mellitus, lupus erythematosus, allo-antibodies from enzyme replacement therapy, drugs (heroin, lithium), viral infections, amyloidosis and paraproteinemias etc. These factors aligned in a sequence:
Diabetes Mellitus
Lupus erythematosus.
Viral infections (e.g., hepatitis B, hepatitis C, human immunodeficiency virus [HIV]).
Amyloidosis and paraproteinemias.
Preeclampsia [14].
Dyslipidemia and NS
Many patients with renal disorders such as NS have one of most frequent changes include metabolism of lipoprotein and then quantitatively and qualitatively changed the lipid nature and affect degree of renal disorders [1][15]. Hyperlipidemia is a common feature of dyslipidemia in NS, which is marked by increase in total cholesterol, LDL, VLDL and low/ normal HDL [12]. In addition, NS alters lipoproteins composition such as the ratios of cholesterol/triglycerides, free cholesterol/cholesterol esters, and phospholipids/proteins are significantly increased [6]. Hyperlipidemia in NS is caused by two factors, whether it stimulates liver protein synthesis that resulting in the overproduction of lipoproteins TG and TG-rich lipoprotein, or the catabolism of lipid is decreased due to lower lipoprotein lipase levels mainly involved in lipoprotein breakdown. Otherwise, apolipoprotein C2 is a cofactor, which is also lost by increased proteins filtration [16]. Animal studies demonstrated that the hyperlipidemia is linked with increased activity of hepatic Acyl CoA, acyl-CoA carboxylase (ACC), diacylglycerol acyltransferase (DGAT-) and fatty acid synthase (FAS), the key catalytic enzymes in the final step in triglyceride and fatty acid metabolism [17],[18]. Limited data on the mechanisms involved in dysregulation of hepatic fatty acid metabolism and its involvement in NS checked the exact understanding. Therefore, many studies were conducted to investigate the expressions and activities of some lipoproteins involved in regulation, synthesis and catabolism of fatty acid and cholesterol in the liver of NS patient. Liver plays a critical role in homeostasis of fatty acid and triglyceride (TG) metabolism. Fatty acid metabolism in hepatocytes comprised of following four main steps:
Absorption of free fatty acids derived from phospholipids and triglycerides hydrolysis by hepatic lipase, and endocytosis of the chylomicron.
de novo synthesis of fatty acid,
Fatty acid catabolism can be accomplished through mitochondria oxidation, peroxisomes, and endoplasmic reticulum.
Utilization of fatty acid in synthesis of triglyceride and its incorporation in VLDL for release in the plasma [19],[20].
Many studies have shown that NS results in impaired removal of TG-rich lipoproteins, VLDL and chylomicrons. Due to lower lipoprotein lipase activity which down-regulates primary pathway of TG-rich lipoprotein and VLDL receptor in the muscle and adipose tissues, while hepatic triglyceride lipase in liver tissues [21],[22].
Plasma lipid and lipoprotein Metabolism
In the plasma, lipids are carried by soluble lipoproteins, which consist of two parts a nonpolar lipid core (triglycerides, cholesterol esters) and a polar lipids envelope such as apolipoproteins (apo) and phospholipids [23]. Various classes of plasma lipoproteins have been stated based on their ultracentrifugation characteristics such as high-density HDL, low-density LDL, intermediate-density IDL, and very-low-density VLDL [24]. VLDL serves as carrier and transporter of fats (triglycerides and cholesterol) from intestine and liver (the sites of absorption) to the peripheral tissues (myocytes, steroidogenic glands etc.) or to storage site i.e., adipocytes. On the contrary, HDL serves as a vehicle to transport the fats from peripheral tissues to the liver or steroidogenic organs such as adrenals, ovary and testes. HDL is crucial in metabolism of triglyceride-rich lipoproteins. Moreover, HDL acts as a potent endogenous inhibitors for inflammation, platelet adhesion, and LDL oxidation [25]-[27].
Methods
Data included 21 cases of children with nephrotic syndrome between 4-15 years of age obtain from Abn al-baladi hospital and 21 cases of healthy children were taken as control group.
Reagents and Enzymes Methods
Cholesterol Reagent
Reagent (A): (Tris pH 7.4 92 mmol/L and Phenol 0.3 mmol/L )( bio system); o-Dianisidine Solution (ODA) ; KOH ; Potassium Phosphate; Peroxidase Enzyme Solution (POD) ; and Cholesterol Oxidase Solution (Sigma-Aldrich) ; Cholesterol standard 5.17 mmol/L (bio system).
Reagents for Determined HDL level
Reagent (A) phosphotungstate 0.4mmol/L, magnesium chloride 20mmol/L; Reagent (B) Phosphate 35 mmol/L,cholesterol esterase02U/mL, cholesterol oxidase 0.1U/mL, peroxidase 1 U/ml,40-aminoantipyrine 0.5 mmol/L, sodium chloride 0.5 mmol/L, di chlorophenol sulfonate 4 mmol/L Reagent (C) HDL standard 15 mg/ml.
Blood Collection
Fasting blood samples are collected from child with and without any clinical and laboratory findings of nephrotic. For lipid measurements, serum was used in preference to plasma to avoid the diluting effect of anticoagulants, which results in about 3% difference in concentrations. Nevertheless, some countries may have special reasons to use plasma, e.g. to retain comparability with the earlier surveys.
Separation of Serum or Plasma
After centrifugation, the tubes should be inspected carefully in order to recognize possible hemolysis. If vacuum gel tubes are used, it should be checked that the gel surface is straight, the layers are properly separated, there are no red cells above the gel surface, there are no fibrin filaments in the sample and the sample is not coagulated after the centrifugation. If the serum samples are pooled the hemolyzed samples should be kept separate.
Biochemical Assay
All parameters were assayed using standard methods (biosystem Laboratories, U.K) total cholesterol, triglycerides, HDL-cholesterol, while VLDL cholesterol was estimated by calculations LDL-cholesterol.
Determination of Cholesterol and triglyceride levels in serum
All samples were pipette into the cuvettes as following:
Table 1: Method of cholesterol and triglyceride measurement
| Reagent /serum | Volume (µl) Test | Volume (µl) blank | Volume (µl) Standard |
| Serum | 10 | —– | —– |
| Reagent A | 1000 | 1000 | 1000 |
| Reagent B | —– | —– | 10 |
All samples were mixed well, and then incubated for 5 min at 37°C. The change in absorbance at 500nm was recorded and the ratio of absorbance at 500/time (minutes) was obtained by using the maximum linear. The reaction color was stable for 30 min. Read the absorbance of the test and standard against blank [28].
Calculation the concentrations of cholesterol and triglyceride in mg/ml: (absorbance of test/ absorbance of stander) X 200.
Determination the Concentration of HDL-cholesterol
Exact amounts from reagent and sample were mixed well as (table 1.2) and allow the reaction mixture to stand for 10 minutes at room temperature, Centrifuge at 4000 r.p.m for 10 minutes to obtain a clear supernatant.
Table 2: Method of of HDL-cholesterol measurement
| Reagent /serum | Volume (µl) Test | Volume (µl) blank | Volume (µl) Standard | |||
| Serum | 10 | ——- | —— | |||
| Reagent A | 1000 | 1000 | 1000 | |||
| Stand the reaction at room temperature for 10 min, then centrifuged the reaction for 10 min at 4000 rpm | ||||||
| Distilled water | —————— | 50µL | —————– | |||
| R3 HDL standard | ——————- | —————— | 50µL | |||
| Supernatant | 50 µL | —————— | – | |||
The Mixture, incubated for 10 min. at 20-25ºC. The absorbance at 500nm of the test A(T) and standard A(S) were read against reagent blank.
Calculation HDL Cholesterol = ab(Test) / ab (standard) X concentration of HDL standard X dilution factor.
Determination the Concentration of VLDL and LDL
VLDL) = Triglycerides/5
LDL = Total Cholesterol – HDL – (Triglycerides/5)(21)
Statistical Analysis
The Statistical Analysis System-(22) program was used to study the effect of difference parameters. Least significant difference –LSD test was used to significant compare between means in this study.
Results and Discussion
We selected a group of 42 Iraqi children ages 4-15 years, of which 21 Normal and 21 Nephrotic syndrome were included. The normal or control group (23) patients were never have reported any pathological history. The comparison between serum total cholesterol, TG, HDL and VLDL was studied (Table 2) and correlation was found in serum lipid levels of Nephrotic syndrome.
Table 3: Observed Serum Levels of lipoproteins between the two groups.
| The Group | Mean ± SE (mg/l) | ||||
| Cholesterol | Triglyceride | HDL | LDL | VLDL | |
| Healthy (Control) | 98.55 ± 2.23 | 89.00 ± 5.61 | 52.00 ± 2.39 | 42.00 ± 2.63 | 18.00 ± 1.07 |
| Patients with Nephrotic syndrome | 103.25±3.79 | 228.00 ± 21.74 | 48.00 ± 1.62 | 211.00 ± 18.03 | 46.00 ± 1.25 |
| LSD value | 8.891 NS | 37.057 ** | 7.822 NS | 32.209 ** | 9.041 ** |
| ** (P<0.01). | |||||
The data in (Figure 2) showed a significant increase in TG, LDL and VLDL levels. Cholesterol levels showed no significant change. Our patients had increased concentrations of triglycerides, VLDL and LDL fractions of serum. These results, suggest that the abnormalities in serum fractions in patients with the nephrotic syndrome are due to increased numbers of lipoproteins particles, as have been described in [24] An increase in the level of glycerides in nephrotics patients might due to down regulation of lipoprotein lipase, in a very mimicking way in nephrotics of skeletal muscles, myocardium and adipose tissue [32].
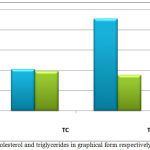 |
Figure 2: Cholesterol and triglycerides in graphical form respectively (mean±SD).
|
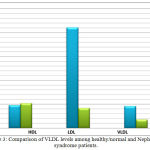 |
Figure 3: Comparison of VLDL levels among healthy/normal and Nephroitics syndrome patients.
|
Furthermore, increased levels of total cholesterol and HDL in nephrotic patients were seen but the changes were not significant compared to control group. Although most investigators have found increased concentrations of HDL in patients with the nephrotic syndrome, the results of HDL levels have been contradictory, with other studies. The mechanism has been postulated that HDL cholesterol does not increase in these patients because it is lost in the urine. For instance, many studies reported an elevated level of HDL-C in nephritic patients [24] Such variation in data is primarily due to the history of patients or possibly the nephrotic syndrome is accompanied by other systemic disorders such as renal failure, diabetes mellitus (or both), and type of drug applied e.g. corticosteroids bearing confounding effects on the lipoprotein patterns. In addition, the incidence of nephrotic syndrome varies due to climatic factors and various other factors particularly change in food, life style, climate, genetic traits and ethnic origin [33],[34].
Conclusion
Result has shown that hyperlipidemia is one of the common features of nephrotic syndrome. The level of cholesterol in the serum of NS patients reaches normal value at the verge of therapy. Furthermore, maintaining the level of lipid in the body is essential for a good health. However, any abnormalities in lipid levels, as is the case of relapse, lead to atherosclerotic cardiovascular disease, thrombo embolic complications, and lipid accumulation in glomeruli and proximal tubular epithelial cells. Such negative consequences cause in return to develop renal failures. Therefore, it is recommended that full lipid profile should be checked and stated in patients with suspected NS. Such a procedure helps prevent any further complications-based syndrome.
Future Scope
It is highly recommended to Investigate specific lipoproteins to direct treatment toward inhibiting LDL and TG; and Conducting multicenter clinical studies to improve current therapies and prevent acute and long-term complications.
Acknowledgment
I’m so grateful to the anonymous reviewers for their assessment of the study and great thanks to families of patients for help me to get samples
Conflict of Interest
None
Funding Source
Self- funded project
Reference
- Bagga A.; Mantan; M. (2005): Nephrotic syndrome in children. Indian J Med Res 122(1): 13-28.
- Tryggvason K, Patrakka J, Wartiovaara J (2006) Hereditary proteinuria syndrome and mechanism of proteinuria. N Engl J Med 354(13): 1387-1401.
CrossRef - Mc Kenny PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM (2001) Time trends and ethnic patternsof childhood nephritic syndrome in Yorkshire, UK. Pediatr Nephrol 16(12):1040-1044.
CrossRef - Priya P. and Ellis D. (2015): Nephrotic syndrome. Nelson Textbook of Pediatrics, (20th edn), Philadelphia, USA, WB Saunders, pp. 2521-2523. 8.
- Murray R. ;Granner D and Mayes P. (2000): In Harper’s Biochemistry, (25th edn) international edition Appleton & Lange pp. 740-741.9.
- Nosratola D. (2016): Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences, International Society of Nephrology Volume 90, Issue 1, Pages 41–52.
- Haraldsson B, Nyström J, Deen WM. (2008):Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 88(2):451-87.
CrossRef - Russo LM, Bakris GL, Comper WD.(2002): Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 39(5):899-919.
CrossRef - Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW. (2001): Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 60(5):1885-92.
CrossRef - Hamm L. and Batuman V.(2003): Edema in the nephrotic syndrome: new aspect of an old enigma. J Am Soc Nephrol. 14(12):3288-9. [Medline].
CrossRef - Freedberg, Irwin M. ed. (2003). Fitzpatrick’s dermatology in general medicine. (6th ed.). New York, NY [u.a.]: McGraw-Hill. p. 659. ISBN0-07-138076-0.
- Jyotish P. and Chandra P.(2016): Lipid Profile Abnormalities in Nephrotic Syndrome ;Asian Journal of Biomedical and Pharmaceutical Sciences, 6(54), 17-19.
- Niaudet P.(2004): Genetic forms of nephrotic syndrome. Pediatr Nephrol 19 : 1313-8.
CrossRef - Beins N.T and Dell K.M. (2015): Long-Term Outcomes in Children with Steroid-Resistant Nephrotic Syndrome Treated with Calcineurin Inhibitors. Front Pediatr.. 3:104.
CrossRef - Kronenberg F. (2005): Dyslipidemia and nephrotic syndrome: recent advances. J Ren Nutr. 15(2):195-203.
CrossRef - García, J.; Merino J. and González J. (1995): Fisiopatología glomerular”. Patología General. Semiología Clínica y Fisiopatología. McGraw – Hill Interamericana. ISBN 8448600932.
- Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M.(2005): Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. ;280:32317–32325.
CrossRef - Zhou Y.; Zhang X.; Chen L.; Wu J.; Dang H.; Wei M, Fan Y, Zhang Y, Zhu Y, Wang N, Breyer MD, Guan Y. (2008): Expression profiling of hepatic genes associated with lipid metabolism in nephrotic rats. Am J Physiol Renal Physiol. ;295:F662–71.
CrossRef - Vaziri ND.(2003): Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. ;63:1964–1976.
CrossRef - Vaziri ND, Kim CH, Phan D, Kim S, Liang K.(2004): Up-regulation of hepatic Acyl CoA: Diacylglycerol acyltransferase-1 (DGAT-1) expression in nephrotic syndrome. Kidney Int. 66:262–267.
CrossRef - Friedewald WT, Levy RI, Fredrickson DS.(1 972):Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18:499–502.
- (2012): Statistical Analysis System, User’s Guide. Statistical. Version 9.1th ed. SAS. Inst. Inc. Cary. N.C. USA.
- Adu E.M (2013): Serum lipid profile abnormalities among patients with nephrotic syndrome. International Journal of Medicine and Biomedical Research. ;2(1):13-17.
CrossRef - Liang K.and Vaziri ND.(1997): Acquired VLDL receptor deficiency in experimental nephrosis. Kidney Int.;51:1761–1765.
CrossRef - Sato T.; Liang K. and Vaziri ND. (2002): Downregulation of lipoprotein lipase and VLDL receptor in rats with food glomerulosclerosis. Kidney Int. 61:157–162.
CrossRef - Vaziri N.(2006): Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences, Am J Physiol Renal Physiol 290: F262–F272.
CrossRef - Gibbons GF, Wiggins D, Brown AM, Hebbachi AM (2004): “Synthesis and function of hepatic very-low-density lipoprotein.”. Biochem Soc Trans. 32(Pt 1): 59–64.
CrossRef - Navab M.; Berliner J.; Subbanagounder G.; Hama S.; Lusis A.; Castellani L.; Reddy S.; Shih D.; Shi W.; Watson A.; Van Lenten B.; Vora D. and Fogelman AM. (2001): HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol 21: 481–488.
CrossRef - Genest J ; Marcil M; Denis M; and Yu L.(1999): High density lipoproteins in health and in disease. J Investig Med 47: 31–42.
- Huang CX, Zhang YL (2013). “The target of regulating the ATP-binding cassette A1 protein (ABCA1): promoting ABCA1-mediated cholesterol efflux in different cells”. Current Pharmaceutical Biotechnology. 14(6): 623–31.
CrossRef - Bergmeyer, H.U.,Gawehn, K. and Grassl, M. (1974): in Methods of Enzymatic Analysis (Bergmeyer, H.U. ed) Volume I, Second Edition, 457-458, Academic Press, Inc., New York, NY.
- Vaziri ND, Sato T, and Liang K.(2003): Molecular mechanism of altered cholesterol metabolism in focal glomerulosclerosis. Kidney Int 63: 1756– 1763.
CrossRef - Ruggenenti P. ; Ruggiero B.; Cravedi p.; Vivarelli;Massella L.; Marasà M.; Chianca A.; Rubis N.;Ene-Iordache B.; Rudnickihttp://jasn.asnjournals.org/content/25/4/850 – aff-4 M.;, Pollastro R.; Capasso G.; Pisani A.;Pennesi M. and Emma F.(2014) Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol.. 25 (4):850-63.
- Krishnaswany D , Indumati V , Satihkumar D ,Viijay V, Maharudra S , Amareshwara M and Rajeshwari V.(2011): Serum proteins, initial and follow-up lipid profile in children with nephrotic syndrome. IJABPT ,2:59-63.








