Golsa Salehimehr1, Fatemeh Baladi2 and Hanif Allahbakhshi3
1Department of Endodontics, Kashan University of Medical Sciences, Kashan, Iran.
2Department of Oral Diseased and Diagnosis, Babol University of Medical Sciences, Babol, Iran.
3Department of Prosthodontics, Kashan University of Medical Sciences, Kashan, Iran.
Corresponding Author E-mail: h.allahbakhshi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1212
Abstract
Pro Root MTA has many uses in endodontic therapy but is limited by its difficult handling characteristics. X-ray diffraction analysis was used to characterize and identify crystalline phases, and energy dispersive x-ray spectrometer was used to determine the chemical composition of the test materials. Setting time was measured by Gilmore in 5 minutes. The pH of the materials was measured after mixing. The concentration of calcium ions was obtained through atomic absorption spectroscopy technique. In addition, compressive strengths of the test materials were measured by modifying the previous method. Data were compared by analysis of variance and and the Student's test (t-test). There were differences in the chemical composition and crystalline structures between the powder and set forms of any of the NERM and Pro Root MTA. Setting time in NERM and Angelus MTA samples was 25 and 14 minutes, respectively (P<0.05). The compressive strength values of NERM were greater than the Pro Root MTA. Both materials tested were alkaline and released calcium, the results revealed a higher pH for Pro Root MTA. Current findings have strongly suggested that the favorable chemical and physical properties are exhibited by NERM, but Pro Root MTA is preferred in terms of setting time. With more studies and considering the physical and chemical properties of NREM, it could be recommended for clinical application.
Keywords
Pro Root MTA; New Endodontic Restorative Material; PH; Setting Time
Download this article as:| Copy the following to cite this article: Salehimehr G, Baladi F, Allahbakhshi H. Physical and Chemical Properties of New Endodontic Restorative Material in Comparison with Pro Root MTA. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Salehimehr G, Baladi F, Allahbakhshi H. Physical and Chemical Properties of New Endodontic Restorative Material in Comparison with Pro Root MTA. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16735 |
Introduction
An ideal endodontic repair material would adhere to tooth structure, maintain a sufficient seal, be insoluble in tissue fluids, dimensionally stable, non-resorbable, radiopaque, and exhibit biocompatibility.1,2 A number of materials have historically been used for retrograde fillings and perforation repair, such as amalgam, composite resin, glass-ionomer and zinc-oxide-eugenol cements.1,3 Unfortunately, none of these materials have capableto satisfy the total requirements of an ideal material.1,2 Mineral trioxide aggregate (MTA) is a biomaterial that has been investigated for endodontic applications since the early1990s. MTA was first described in the dental scientific literature in 1993 and given approval for endodontic use by the U.S. Food and Drug Administration in 1998.4,5
Originally, MTA products required a few hours for the initial and final setting, which is uncommon in dental materials. MTA can be a difficult material to use because it is perceived as coarse, sets slowly, and is easily washed out of a moist site. Special delivery systems do not overcome these difficulties. Newer materials are available that set more quickly and have added characteristics.6
Pro Root MTA introduced as a refined, medical grade root repair material that meets dental standards (ISO 9917) for purity and performance (ISO 6876 and ADA 57). The primary components of Pro Root MTA include tricalcium silicate and dicalcium silicate naturally composed, bismuth oxide (for radiopacity), tricalcium aluminate (C3A) and tetracalciumaluminoferrite (C4AF). By composition, Pro Root MTA contains: 55% (C3S), 19% (C2S), 10% (C3A), 7% (C4AF), 2.8% MgO, 2.9% (SO3), 1.0% Ignition loss and 1.0% free CaO.7-9 Pro Root MTA does not contain calcium phosphate, calcium oxide or silica. Calcia, alumina and silica are the primary ingredients of portland cement. The typical portland cement contains about only 0.2% of phosphate. Pro Root MTA was developed for reducing discoloration to the tooth structure. It eliminated C4AF (tetracalciumaluminoferrite) as iron (Fe)’s main discoloration induced element.; The neurotoxicity of calcium aluminate compounds has not been demonstrated; However some studies have shown an increased risk of developing Alzheimer’s disease with environmental factors such the intake of metals, particularly aluminium.9-11
Here is the time to use the materials with desirable properties, therefore designing and manufacturing materials with desirable properties which do not carry ProRoot MTA problems is major goal.12-14
Reconstructive products that were produced based on tri-calcium silicate; with different physical and chemical properties have its own advantages and disadvantages. Previous studies have promoted additives to shorten working time, modify MTA’s handling properties, and prevent washout.12,15
The new calcium silicate materials have been reported with improved working characteristics,16,17 but evidence is still lacking to support these materials as improvements from MTA. Recently, a new restorative material has been produced as NERM in Iran that partly has the MTA physical and chemical properties. In current study we decided to compare the new endodontic restorative material in Iran (NERM) with Pro Root MTA in terms of physical properties such as setting time, compressive strength and chemical properties such as pH, the release of calcium ions, the final phase of each of these products and the structure of matter.
Materials and Methods
In this experimental study, the Pro Root MTA (Dentsply, Tulsa Dental, Tulsa), which was composed of 75% Portland cement, 5% calcium, and 20% bismuth oxide, and the New Endodontic Restorative Material (NERM) (Tehran, Iran) were prepared. NERM’s powder component is consisting of Portland cement, bismuth oxide and its liquid component is consisting of Na2HPO4 solution. The Portland cement is fully mixed with bismuth oxide in the ratio of 3 to 1 (i.e., 75 % Portland cement and 25 % bismuth oxide) for preparation of the NERM powder component. The liquid part containing the 0.1 M Na2HPO4 solution that is prepared by dissolving the 0.126 g Na2HPO4 in 10 mL distilled water. Then 5 samples were prepared based on ISO 2001 68762 standards to check the physical and chemical properties of material.14
XRD Analysis
The X-ray diffraction (XRD) was used to identify and characterize crystal phases. The prepared 5 samples materials were mounted onto the XRD apparatus (Geigerflex Horizontal diffractometer with a graphite crystal monochrometer; Rigaku/MSC, Woodlands, TX). The x-ray beam angle 2 range was set between 3 degrees (3000) to 70 degrees (70000) and scanned at 2 degrees per minute. The peaks on the diffraction pattern were marked using the Rigaku software (version 2.8). The Cu x-ray source was set at accelerating voltage of 45 KV and the current in the electron beam at 30 mA and on continuous scan mode. Then the peaks were compared and matched with that of the standard material in the powder diffraction file (JCPDS International Center for Diffraction Data 1998, Pennsylvania) using a micro powder diffraction search and matching analysis program.
Microscopic Survey
Each samples powder (n=5) was placed on gold-coated aluminum stop. Analytical scanning electron microscopy was performed on JEOL 6400 SEM (Tokyo, Japan). The microscope was equipped with an Oxford energy dispersive x-ray spectrometer (EDS) and wavelength dispersive x-ray spectrometer (WDS). The EDS system was used to determine the chemical composition of the examined materials.
Compressive Strength
The compressive strengths of the test materials (n=5) were determined by modifying the method recommended by the BSI (18). Custom made cylindrical delrin molds 12 mm in length and 6 mm in diameter were used, instead of stainless steel molds as recommended in the BSI, to prepare the specimens for the compressive strength tests. The strength of the materials was determined at 1, 3, 7 and 14 days after mixing using a Universal Testing Machine (Instron, Model 1334, Instron Corp., Canton, MA). The maximum fracture load for each specimen was measured and recorded and the compressive strength was calculated in megapascals according to the formula
C=4P ⁄ πD2
where P is the maximum load applied in Newton and D is the mean diameter of the specimen in millimeters. Statistical analysis was carried out using t-test and Fisher’s LSD at the 0.05 level of significance.
Setting Time
The setting time was determined according to the method described by ASTM C266-03 (19), which requires the measurement of both initial and final setting times using the initial and final Gillmore needles, respectively. The initial and final setting times of the materials (n=5) were determined according to these recommendations. The setting times for each material were measured four times. Statistical analyses were carried out for setting time using t-test and Fisher’s LSD at 0.05 level of significance.
pH
The pH of the materials (n=5) as they set was measured with a pH meter (Orion PerpHect Log R meter, Model 370, Orion Research Inc., Boston, MA) using a temperature-compensated electrode. The readings were taken periodically every 2 min from the start of mixing for 60 min, and then after 24 h. Then repeated three times for each material and the mean pH at each time interval was plotted against time. Statistical analysis was carried out using t-test and Fisher’s LSD at the 0.05 level of significance at three time points, namely, when the cement was freshly mixed, at 30 min and at 60 min.
Calcium Ion Release Analysis
A total of 5 samples were used for each material. Each tube was sealed in a flask containing 10 mL of distilled water. The amount of calcium released into the deionized water was determined respectively at 5 min, 1 and 24 hours after spatulation. After each measurement, the tubes were moved to new flasks with fresh deionized water. The measurements were performed with the aid of an atomic absorption spectrophotometer (Model GBC 904; CG Corp, Melbourne, Australia) equipped with a hollow cathode calcium lamp under the following operating conditions: Lamp current: 3 mA, Fuel: acetylene, Support: oxygen, Stoichiometry: reducing, Wavelength: 422.7 nm and Slit: 0.2 nm. To prevent possible interference by phosphates and alkaline metals, all glassware was prewashed with 5% nitric acid. A standard solution of 10 mg/dL of calcium was diluted in 10% EDTA to obtain 0.025, 0.05, 0.1, 0.2, and 0.3 mg/dL concentrations. To calibrate the apparatus for zero absorbance, 10% EDTA was used as the blank. The samples were diluted as necessary to perform the evaluation. The results were calculated by using the equation of the standard curve line.
Results
XRD Analysis
The crystal structure of Pro Root MTA and NERM were similar (Fig. 1). Both materials were composed mainly of bismuth oxide crystalline structure and calcium silicate oxide. For each of the materials, there were no noticeable differences in the crystalline structure between them.
 |
Figure 1: X-ray diffraction patterns of ProRoot MTA and NERM showing peaks representing the crystal phases present in each material.
|
Microscopic Survey
The microstructures of the samples were examined by electron microscopy at three different magnifications. The results showed no significant differences in the microstructure of the two materials [Fig 2].
 |
Figure 2: Microstructure of Pro Root MTA and NERM by electron microscopy (X 250).
|
Setting Time
Setting time in NERM samples was about 25 minutes and in Pro Root MTA was about 14 minutes (P<0.05).
Compressive Strength
The compressive strength values of NERM and Pro Root MTA at different times was shown in fig 3.The compressive strength values of NERM were greater than the Pro Root MTA at 14 days.
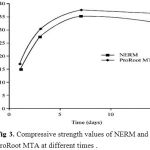 |
Figure 3: Compressive strength values of NERM and ProRoot MTA at different times.
|
pH
The mean pH values recorded for the materials at the different periods tested are summarized in Table 1. The values for pH were lower during the 5 min, 1 and 24 hours for NERM when compared with Pro Root MTA. The pH values recorded for ProRoot MTA were higher at all time periods.
Table 1: pH values recorded at different time periods (mean ± SD)
| Materials | 5 min | 1 hour | 24 hours |
| ProRoot MTA | 9.62±0.3 | 11.04±0.24 | 11.68±0.39 |
| NERM | 8.71±0.4 | 10.01±0.63 | 11.23±0.71 |
| P value | P<0.01 | P<0.01 | P=0.4 |
Calcium Ion Release Analysis
Table 2 presents the mean calcium release at the different time periods. The values for calcium release were higher during the 5 min and 1 hour for NERM, after which it tended to decrease in 24 hours for NERM in comparision with Pro Root MTA. Statistically significant difference was reported.
Table 2: Calcium released (mg/dL) recorded over different periods of time (mean ± SD)
| Materials | 5 min | 1 hour | 24 hours |
| ProRoot MTA | 0.09±0.02 | 0.2±0.04 | 0.59±0.13 |
| NERM | 0.3±0.06 | 0.35±0.11 | 0.29±0.08 |
| P value | P<0.001 | P<0.02 | P<0.002 |
Discussion
The Pro Root MTA patent description states that MTA is a Type 1 ordinary Portland cement, and a bismuth oxide is added for dental radiologic diagnosis.20 Saidon10 reported that both Pro Root MTA and Portland cement had similar physical, chemical, and biologic properties, and the biocompatibility of both materials was due to the similarity in constituents. Funteas21 reported in 2003 that no difference was found in the presence of 14 elements between Pro Root MTA and Portland cement except for the bismuth that was present in MTA.
Although various types of materials are available for use as a root filling, there is no consensus on choosing the best material as filler. Portland cement has been used as the main component in the construction of dental material. There are a lot of knowledge and information about Portland cement that can be used to improve and make better dental material.16
Adding of materials such as metal and aluminate oxides to cement that reduces the setting time or increase their strength can have the same effect on MTA and Portland cement. However, there are reports that show the cement workers suffering from respiratory and visual problems which are the result of alkali dust that is scattered in cement production process. Inflammation, shortness of breath and bronchitis are the other long-term effects of Portland cement. So more care should be done in selection of Portland cement for intraoral use that be consistent with human body and do not harm it.5,22
MTA is a successful improved sample of Portland cement that could get FDA approval in vivo and in vitro tests and could prove itself as compatible with the human body. Also in NERM samples, the use of phosphate solution as liquid phase can be formed a similar phase and mineral hydroxyl within human body. They can be effective in a better biological adaptation toward Pro Root MTA. This theory requires further biological tests.14
Results showed that XRD pattern in NERM and Pro Root MTA samples are fairly similar, which indicates that these materials have the same crystal structure. Bismuth oxides are added to dental cement due to the characteristics of opaque that are also observed in these compounds. In all cases the formation of calcium silicate hydrate is observed in 29.3, which corresponds to Portland cement hydration and cause increasing the rate of hydration and reducing the setting time. Experiments showed that the present elements (tri-calcium silicate, tri-calcium aluminate and calcium silicate) were very similar to each other and the main ingredient in cement manufacturing and Pro Root MTA are equal.7,23
Two substances, NERM and Pro Root MTA samples, have been evaluated in this study and show the release of calcium ions and PH changes. During the first three hours, its amount is increased and the speed of released calcium ions was rising. According to Pro Root MTA samples, in all time intervals, these values were slightly higher and this may be due to large quantities of Portland cement or the release of calcium factors. The achieved alkaline environment is one agent for the treatment of soft and mineral tissue. PH greater than 9 can inactivate the bacteria cell wall.9,24
NERM samples In Figure 2, shows angular and some needle -shaped particles that have surface and are porous. The role of networks and porous particles is important during hydration reaction. When the powder is mixed with water, a special structure of the network is created. When the powder and liquid phases were combined in a reasonable amount, they stuck to each other by participating in the gluing process and they became hard at room temperature.6
The compressive strength is not so important for root filling because this type of root filling materials do not bear the direct burden.25 NERM sample stability increases over time and reaches about 35 MPa after 7 days and after that it is relatively fixed in advance.
Setting time is one of the important clinical factors, because the prolonged setting time of cement, cause consistency and reduced ability to maintain stability in the shape of cement in oral environment, especially in the presence of solvents. Reduction of setting time makes it difficult to use. The proper setting time is considered as 10 and 15 minutes.23 Ratio of solid to liquid phase has also effects on the cement strength and setting time. The greater amount of liquid phase cause the less in the viscosity of cement and this has influence on the setting time. The final result is a reduction in strength. Cement setting and hardening reactions take place over time and creates links between hydrate components. Generally setting time of Portland and MTA cement has two stages. First, after mixing the powder and water, the dipping reaction in silicates starts and a gel containing of calcium silicate hydrates is formed and calcium hydroxide is released. In the next step, calcium hydroxide gradually reacts with other minerals and other hydrated compounds are created. Calcium silicate is the main factor in the connection of crystalline calcium hydroxide. Tri-calcium aluminate plays an important role in the cement setting.15,23,24 NERM and ProRoot MTA Samples have setting time of about 25 and 14 minutes, respectively. This result shows that the NERM requires more time for setting.
Conclusion
The physical and chemical properties of NERM and ProRoot MTA materials have been discussed. There were no noticeable differences in the composition and crystalline structure between ProRoot MTA and NERM samples. The results revealed a higher pH for MTA ProRoot than NERM. NERM setting time and calcium ions release is more than the ProRoot MTA. So further studies need to confirm the NERM properties and in near future, it could be recommended for clinical application.
Acknowledgement
The authors of this study thank all participants that co-operated in this project; your dedication is deeply appreciated. This project was financially supported by a grant from Kashan University of Medical Sciences, Kashan, Iran.
Declaration of Interest
The authors of this manuscript have no invested interests in products described or used in this study. The authors have no conflicts of interest.
References
- Johnson B. R. Considerations in the selection of a root-end filling material. Oral surgery, oral medicine, oral pathology, oral radiology and endodontics. 1999;87(4):398-404.
CrossRef - Kratchman S. I. Perforation repair and one-step apexification procedures. Dental clinics of North America. 2004;48(1):291-307.
CrossRef - Bryan E. B., Woollard G., Mitchell W. C. Nonsurgical repair of furcal perforations: a literature review. General dentistry. 1999;47(3):274-8. quiz 9-80.
- Camilleri J., Ford T. R. P. Mineral trioxide aggregate a review of the constituents and biological properties of the material. International endodontic journal. 2006;39(10):747-54.
CrossRef - Malhotra N., Agarwal A., Mala K. Mineral trioxide aggregate: a review of physical properties. Compendium of continuing education in dentistry (Jamesburg, NJ : 1995). 2013;34(2):e25-32.
- Rao A., Rao A., Shenoy R. Mineral trioxide aggregate–a review. The Journal of clinical pediatric dentistry. 2009;34(1):1-7.
CrossRef - Schmitt D., Lee J., Bogen G. Multifaceted use of ProRoot MTA root canal repair material. Pediatric dentistry. 2001;23(4):326-30.
- Lamb E. L., Loushine R. J., Weller R. N., Kimbrough W. F., Pashley D. H. Effect of root resection on the apical sealing ability of mineral trioxide aggregate. Oral surgery, oral medicine, oral pathology, oral radiology and endodontics. 2003;95(6):732-5.
CrossRef. - Duarte M. A., Demarchi A. C., Yamashita J. C., Kuga M. C., Fraga Sde C. pH and calcium ion release of 2 root-end filling materials. Oral surgery, oral medicine, oral pathology, oral radiology and endodontics. 2003;95(3):345-7.
CrossRef - Saidon J., He J., Zhu Q., Safavi K., Spangberg L. S. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral surgery, oral medicine, oral pathology, oral radiology and endodontics. 2003;95(4):483-9.
CrossRef - Shcherbatykh I., Carpenter D. O. The role of metals in the etiology of Alzheimer’s disease. Journal of Alzheimer’s disease. JAD. 2007;11(2):191-205.
CrossRef - Kogan P., He J., Glickman G. N., Watanabe I. The effects of various additives on setting properties of MTA. Journal of endodontics. 2006;32(6):569-72.
CrossRef - Song J. S., Mante F. K., Romanow W. J., Kim S. Chemical analysis of powder and set forms of Portland cement, gray Pro Root MTA, white ProRoot MTA, and gray MTA-Angelus. Oral surgery, oral medicine, oral pathology, oral radiology and endodontics. 2006;102(6):809-15.
CrossRef - Wiltbank K. B., Schwartz S. A., Schindler W. G. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. Journal of endodontics. 2007;33(10):1235-8.
CrossRef - Bortoluzzi E. A., Broon N. J., Bramante C. M., Felippe W. T., Tanomaru Filho M., Esberard R. M. The influence of calcium chloride on the setting time, solubility, disintegration and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. Journal of endodontics. 2009;35(4):550-4.
CrossRef - Camilleri J. Modification of mineral trioxide aggregate. Physical and mechanical properties. International endodontic journal. 2008;41(10):843-9.
CrossRef - Kao C. T., Shie M. Y., Huang T. H., Ding S. J. Properties of an accelerated mineral trioxide aggregate-like root-end filling material. Journal of endodontics. 2009;35(2):239-42.
CrossRef - British Standards Institution. Specification for Dental Glass Ionomer Cements BS 6039:
- American Society for Testing and Materials. Standard test method for time and setting of hydraulic-cement paste by Gillmore needles. ASTM C. 1981;266–03.
- Torabinejad M., Hong C. U., McDonald F., Pitt Ford T. R. Physical and chemical properties of a new root-end filling material. Journal of endodontics. 1995;21(7):349-53.
CrossRef - Funteas U. R., Wallace J. A., Fochtman E. W. A comparative analysis of Mineral Trioxide Aggregate and Portland cement. Australian endodontic journal the journal of the Australian Society of Endodontology Inc. 2003;29(1):43-4.
CrossRef - Mitchell P. J., Ford T. R. P., Torabinejad M., McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999;20(2):167-73.
CrossRef - Ber B. S., Hatton J. F., Stewart G. P. Chemical modification of proroot mta to improve handling characteristics and decrease setting time. Journal of endodontics. 2007;33(10):1231-4.
CrossRef - Asgary S., Parirokh M., Eghbal M. J., Brink F. Chemical differences between white and gray mineral trioxide aggregate. Journal of endodontics. 2005;31(2):101-3.
CrossRef - Oliveira M. G., Xavier C. B., Demarco F. F., Pinheiro A. L., Costa A. T., Pozza D. H. Comparative chemical study of MTA and Portland cements. Brazilian dental journal. 2007;18(1):3-7.
CrossRef








