Norliana Masbah1, Siti Hawa Nordin2 and Kamsiah Jaarin3
1Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Cheras, Kuala Lumpur.
2Faculty of Medicine, Universiti Sultan Zainal Abidin, Medical Campus, Jalan Sultan Mahmud, 20400 Kuala Terengganu, Terengganu.
3Faculty of Medicine, National Defence University of Malaysia (UPNM), Kem Sungai Besi, 57000 Kuala Lumpur, Malaysia.
Corresponding Author E-mail: kamsiahjaarin@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1201
Abstract
Repeatedly heated cooking oil was reported to have potential detrimental effect on health. Studies have shown that repeatedly heated cooking oil undergoes thermal oxidation which generates reactive oxygen species that contributes to the occurrence of vascular inflammation and dysfunction. These vascular changes lead to multiple health problems such as hypertension, dyslipidemia, atherosclerosis, osteoporosis, as well as kidneys and liver abnormality. Apart from the reactive oxygen species, such abnormality might be due to the reduction of natural antioxidants in the oil with repeated heating. Therefore, there is an inherent need to further explore the individual antioxidants which exert cardiovascular protective effects, particularly via its effects on blood pressure, blood lipid profiles as well as cardiovascular structures. Therefore, this review was undertaken to ascertain if the detrimental effects of heated oil can be reduced by the administration of antioxidants, particularly polyphenols - which are the main focus of this article.
Keywords
Antioxidants; heated oil; polyphenols; cardiovascular; vascular; oxidation
Download this article as:| Copy the following to cite this article: Masbah N, Nordin S. H, Jaarin K. The Role of Antioxidants in Attenuating Heated Oil-Induced Cardiovascular Effects: A Review. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Masbah N, Nordin S. H, Jaarin K. The Role of Antioxidants in Attenuating Heated Oil-Induced Cardiovascular Effects: A Review. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16695 |
Introduction
Repeatedly heated oil undergoes thermal oxidation which generates reactive oxygen species that are detrimental to health. Studies have shown that heated oil induces vascular inflammation and dysfunction (Ng et al. 2012) which have been inflicted in the occurrence of hypertension (Jaarin, Mustafa, and Leong 2011; X.F. Leong et al. 2010). The detrimental effect of heated oil on health is partly due to stress oxidative process that generates reactive oxygen species following the destruction of antioxidants, particularly vitamin E, that occurs with repeated heating (Adam et al. 2007). Reactive oxygen species has been reported to play an important role in the pathogenesis of osteoporosis (Shuid et al. 2007), as well as liver (Jaarin et al. 2009) and kidney abnormalities (Kamisah et al. 2016).
Given that oxidative stress plays an integral part in the pathogenesis of heated oil-induced cardiovascular detrimental effects, it is a reasonable assumption that antioxidant therapy would be effective against this condition. Among various antioxidants, the effects of polyphenols on hypertension and atherosclerosis have been extensively studied. Although the consensus regarding the therapeutic benefits of polyphenols is still unclear (partly due to heterogeneity in polyphenols subclasses as well as the study approach), their potential vascular protective effect shown by many studies are promising. In general, the vascular protective effects demonstrated in several in vivo studies could be summarized as reduction in oxidative stress (de Souza et al. 2010; Natsume and Baba 2013; Scoditti et al. 2012), vascular inflammation (Scoditti et al. 2012; Mukai and Sato 2011) and improvement in endothelial function (Widmer et al. 2013; da Costa et al. 2012).
The role of polyphenols as an antioxidant in food chemistry has been widely established (Quiles et al. 2002; Romano et al. 2013). Therefore, in recent years, polyphenols have been enriched into edible oils to prevent oxidative deterioration of the oil. However, their biological effects to human health, specifically in heated vegetable oil-induced cardiovascular diseases still requires appraisal. Therefore, this review was undertaken to evaluate if the cardiovascular detrimental effects produced by heated oil – in particular blood pressure, dyslipidaemia and vascular damage can be improved or attenuated by the administration of antioxidants. However, due to limited data available for other antioxidants with regards to heated oil-induced cardiovascular effects, this review therefore focused primarily on polyphenols.
Methodology
The literature search was divided into three sections; the first section focused on hypertension, the second section concentrated on blood lipid profiles, while the third section highlighted mainly on cardiovascular structures. For subheading 1, Ovid Medline and Scopus databases were used for articles collection. The search strategy involved a combination of the following sets of keywords (1) heated or therm* or oxidized or oxidized AND (2) vegetable oil* AND (3) hypertens* or blood pressure or vascular or endotheli* and (4) polyphenols or flavonoids. For subheading 2, Ovid Medline and Scopus database were used for articles collection. The search strategy involved a combination of the following keywords (1) heated or therm* or oxidized or oxidized and (2) vegetable oil* and (3) fatty acids or dyslipidemia or hypercholesterolemia and(4) polyphenols or flavonoids or vitamin E. For subheading 3, Ebscohost and Springerlink database were used for articles collection. The search strategy involved a combination of the following keywords (1) heated or therm* or oxidized or oxidized and (2) vegetable oil* and (3) cardiotoxicity or morphology and (4) antioxidants or polyphenols.
Effect of Heated Vegetable Oil on Blood Pressure
The first study which described the detrimental effects of oxidised vegetable oil on blood pressure (Osim, Owu, and Etta 1996) has reported that chronic consumption of diet that contained 15% fresh or oxidised palm oil in animals increased the mean arterial blood pressure and plasma lipid profile compared to the respective control and fresh oil groups. The deleterious effect of thermal-oxidised oil on blood pressure in human was further demonstrated by Soriguer et al. (Soriguer et al. 2003). Leong et al. (X.F. Leong et al. 2010) and Jaarin et al. (Jaarin, Mustafa, and Leong 2011) reported that rats fed with once-, twice, five-times, and ten-time heated palm oil increased blood pressure significantly at the end of the study. The percentage increase in blood pressure (BP) was 5.9%, 23.8%, 24.7%, 25.3%, respectively. The same studies reported that heated once, twice, five-times, and ten-times heated soy oil increased BP with percentage increases of 17.5%, 22.2%, 25.6% and 30.7%, respectively. Heated virgin coconut oil (VCO) (Hamsi et al. 2015) and corn oil (Srijit et al. 2017 in press) have also been shown to increase BP. However, the magnitude of blood pressure raising effect of corn oil was smaller compared to soy oil despite of both being unsaturated oil (Srijit et al. 2017 in press). However, the reason for this was not clear.
Although the blood pressure raising effect of heated oil was reported previously, unfortunately, there are still gaps in understanding the possible mechanisms on blood pressure-raising effect of heated vegetable oil. Currently, few studies have reported the possible mechanism of heated oil induced hypertension. Several studies suggested that heated oil increased vascular reactivity as it caused significant augmentation of vasoconstriction response to phenylephrine and significant attenuation of vasorelaxation response to acetylcholine and sodium nitroprusside (X.F. Leong et al. 2010, 2009). Blood pressure is controlled by vascular endothelium that releases vasodilators such as nitric oxide (NO), prostacyclin (PGI2), and vasoconstrictors including thromboxane A2 (TXA2) and endothelin-1 (ET-1). It was suggested that a disturbance in the production of vasodilators and vasoconstrictors may be attributable to the impaired vasorelaxation and high blood pressure induced by heated oil (X.F. Leong et al. 2009, 2010, 2013). It was reported that heated oil reduced nitrites level (X.F. Leong et al. 2010) and altered thromboxane and prostacyclin production (Ng et al. 2012). Based on these findings, therefore, it was postulated that the lipid peroxidation products present in the heated oil might be the culprit for the increased risk of high blood pressure. The blood pressure raising effect of chronic consumption of thermal-oxidised oils is likely due to vascular inflammation which in turn causes vascular dysfunction. This further leads to an imbalance in the release of vasodilators and vasoconstrictors substances that control vascular reactivity and resistance (Jaarin, Masbah, and Nordin 2016).
Polyphenols and Heated Oil-Induced Hypertension
Endothelial dysfunction and vascular inflammation both augment each other in the pathogenesis of heated oil-induced hypertension; which is evident via dysregulation in vasoactive mediators, inflammatory mediators as well as mechanical function of the vessel (Jaarin, Masbah, and Nordin 2016). Polyphenols have been proven beneficial to vascular function in terms of improvement in blood pressure-regulating enzymes and mediators. It was reported that administration of virgin coconut oil (VCO) in a dose of 1.42ml/kg per day in rats (equivalent to 210 mg/kg per day in human dose) improved plasma level of nitric oxide in five-time heated palm oil treated groups. VCO is known to be one of the polyphenol-rich natural antioxidant. The antioxidant activity of VCO has been reported previously (Marina et al. 2009). Total phenolic content of VCO is estimated to be about 84 mg/100 g oil (Arunima and Rajamohan 2012). The most abundance antioxidants in VCO are phenolic acids comprising of protacatechuic, vanillic, caffeic, syringic, ferulic and p-coumaric acids (Marina et al. 2009). In another study, polyphenol was reported to be able to activate endothelial nitric oxide synthase (eNOS) therefore improves nitric oxide (NO) production (da Costa et al. 2012).
Thromboxane A2 (TXA2) and prostacyclin which are produced by the vascular endothelial cells are important mediators in the pathogenesis of heated-oil induced vascular dysfunction and inflammation. An increase in plasma thromboxane A2 with a reduction in prostacyclin levels due to prolonged intake of heated vegetable oil has been reported in previous studies (Ng et al. 2012; Siti et al. 2017). Flavonoid was also reported to inhibit cyclooxygenase-1 and cyclooxygenase-2 enzymes as well as thromboxane A2 synthase (Jin et al. 2007; Sakata et al. 2003; O’Leary et al. 2004).
Although many studies reported oxidative stress is associated with an increase in Angiotensin II levels, limited studies are available to highlight the specific effects of heated vegetable oil on the regulation of renin-angiotensin-aldosterone system. To the best of our knowledge, prolonged consumption of repeatedly heated palm oil of at least four months duration has the potential to cause elevated levels of Angiotensin Converting Enzyme (ACE) activity (X.F. Leong et al. 2013; Siti et al. 2017). However, in contrast, a shorter animal study lasting for a period of ten weeks has reported otherwise (Yen et al. 2010). In another study, consumption of heated palm oil has been shown to increase blood pressure with associated reduced glomerular filtration rate and renal blood flow (Beshel, Antai, and Osim 2014). Evidently, more studies looking into the effects of heated vegetable oil on the regulation of renin-angiotensin system and renovascular hypertension are needed in order to fill in the gaps of our current understanding. In addition, it was reported that flavonoid-rich citrus leaves extract had the ability to inhibit ACE activity in heated vegetable oil-induced hypertensive rats (Siti et al. 2017). Moreover, many studies have consistently shown that flavonoids were able to inhibit ACE activity (Lee, Lai, and Wu 2015; W. W ang et al. 2014; Oboh et al. 2015).
With regards to vascular function, studies have shown that heated oil augmented vasoconstriction response to phenylephrine while reducing vasorelaxation response to acetylcholine and sodium nitroprusside (X.F. Leong et al. 2009; Nurul-Iman et al. 2013; Siti et al. 2017 ). This impairment in vascular relaxation could be due to the imbalance in vasoactive mediators and enzymes as previously explained which favors vasoconstriction against vasodilation. Although heated vegetable oil has diminished polyphenols content (Andrikopoulos et al. 2002), phenolic acids namely caffeic acid, protocatechuic acid and p-hydroxybenzoic acid which were isolated from a recovery procedure for oil palm phenolics (OPP) were shown to promote vascular relaxation in both aortic rings and perfused mesenteric vascular beds pre-contracted with noradrenaline (Sambanthamurthi et al. 2011). Linking these studies together, it supports the theory that polyphenols supplementation may improve vascular dysfunction which was previously induced by heated vegetable oil. Later studies have also proven that polyphenol-rich VCO attenuated vasoconstriction response to phenylephrine in aortic rings of heated vegetable oil-induced hypertensive rats (Nurul-Iman et al. 2013). In line with this findings, flavonoid-rich citrus leaves extract also showed reduction in vasoconstriction response in aortic rings that were pre-contracted with phenylephrine, although insignificant improvements were seen in both endothelium-dependent and endothelium-independent vasorelaxation processes (Siti et al. 2017). Meanwhile, a human study reported that the intake of polyphenol-rich olive oil daily for at least four months improved endothelial function of early atherosclerotic patients (Widmer et al. 2013). Based on these findings, it is clear that the heterogeneity of polyphenols subclasses possess different vascular relaxation potencies (Loke et al. 2010).
The effects of polyphenols on blood pressure were already reported in previous studies (Gao et al. 2016). Although it is proven that the higher amounts of phenolic compounds showed higher resistance to oxidation (Quiles et al. 2002), it is questionable that the blood pressure-lowering effects observed in heated oil-induced hypertension is solely attributable to the anti-oxidative properties of the polyphenols. This can be explained by the fact that phenolic compounds have quite a low bioavailability profile and are highly metabolised (Morand et al. 2011). The emerging idea is that phenolic compounds may interact with multiple molecular target and signalling pathways in endothelial cells. It is also hypothesised that phenolic compounds are able to induce endogenous antioxidant defence mechanisms, therefore indirectly protecting against oxidative stress (Goszcz et al. 2015).
ADD-X, a polyphenol-rich oil additive has been proven to exert positive effects on blood pressure in rats that were fed heated vegetable oil (Sukalingam et al. 2016b). This study reported that ten times heated palm oil (10HPO) and five times heated palm oil (5HPO) increased blood pressure in ovariectomized (post-menopausal model) rats, with associated increase in thiobarbituric acid reactive substances (TBARS) and significant reduction in antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD) and reduced-glutathione (GSH). Addition of this polyphenol-rich ADD-X to the heated oil significantly reduced TBARS, increased antioxidant enzymes and at the same time decreased the blood pressure-raising effect of heated oil. A study by (Siti et al. 2017) shows that flavonoid-rich citrus leaves extract was able to reduce TBARS while simultaneously increasing serum heme oxygenase-1 (HO-1) levels which is an enzyme that possesses both vasodilator as well as anti-oxidative property.
Heated Oil and its Effect on Blood Lipid Profiles
While increased concentrations of high density lipoprotein-cholesterol (HDL-C) lowers the risk of coronary artery disease (CAD), high plasma low density lipoprotein-cholesterol (LDL-C) concentrations are known to be atherogenic (Després et al. 2001). This is due to the fact that LDL-C have the ability to penetrate into arterial tissues more readily and are also more susceptible to oxidative modification (Pal et al. 2003). In addition to individual lipoprotein or total cholesterol concentrations, the ratio of total cholesterol to HDL cholesterol is considered more crucial in predicting the risk of CAD (Mensink et al. 2003). Although less emphasis is placed on triglyceride (TG) levels in the assessment of CAD risk (Després et al. 2001), high levels of TG in very low density lipoprotein (VLDL) and low levels of HDL-C are typically seen in obesity; which is known to further increase CAD risk (Ebbert and Jensen 2013).
Findings from studies investigating the effects of heated oil on lipid profile were not consistent, as these studies reported diverse outcomes. A few studies reported quite similar outcomes in general, focusing mainly on the levels of total cholesterol, LDL and HDL cholesterol. The first study (Adam et al. 2008) observed the effects of heated soybean oil on serum lipid profile in oestrogen-deficient Sprague-Dawley rats. It was reported that heated soy oil increased serum total cholesterol, LDL-cholesterol and triacylglyceride while simultaneously reducing HDL-C levels after four-months of experiment (Adam et al. 2008). Similarly, another study (Awney 2011) also observed that the male rats fed with thermally oxidised soybean oil had elevated total cholesterol and LDL levels with a reduction in HDL levels. Similarly, a separate study (El-Deen and Eid 2010) also revealed that rats fed with 15% weight/weight (w/w) heated sunflower oil led to a significant increase in their levels of cholesterol, triacylglycerides, LDL-cholesterol and very low density lipoprotein (VLDL) cholesterol with a decrease in HDL lipoprotein levels. In addition, total cholesterol and the esterified cholesterol concentrations in HDL and LDL were significantly higher in repeatedly heated sunflower oil-fed rats. In contrast, Isong et al. (1996) (Isong et al. 1996) which studied the effect of thermal-oxidised palm oil for six months in female and male rats has found that thermal-oxidised oil increases all fat contents of the animal. A human study also reported different outcomes, in that the intake of meal containing deep-fried palm, soybean or olive oils caused a significant elevation in serum triacylglycerides but no changes in total cholesterol, LDL-cholesterol and HDL-cholesterol in healthy men (Rueda-Clausen et al. 2007). Overall, results from these studies suggested that repeated heating of oil resulted in dyslipidaemic changes in blood profiles. However, the precise mechanism by which heated oil cause detrimental effect on lipid profile was poorly understood.
Besides the fact that thermally-oxidised vegetable oil contains lipid peroxidation products which plays an important role in the pathogenesis of dyslipidemia (Adam et al. 2008), fatty acids content in heated oil also contributes to the changes in lipid profiles seen in experimental animals or human subjects. Thermally oxidized oil contains low concentrations of unsaturated fatty acids especially n-3 and n-6 essential fatty acids, however its saturated fatty acids (SFA) and trans-fatty acids contents are considerably high (Serjouie et al. 2010). In general, SFA and trans fatty acids are considered to be hypercholesterolaemic, as both contribute to the rise in LDL-C concentrations. On the other hand, poly-unsaturated fatty acids (PUFAs) family tend to decrease plasma LDL-C and triglycerides respectively (Mensink et al. 2003). However, reports on the effects of heated vegetable oil on plasma free fatty acids (FFA) levels are still inconsistent. For example, Williams et al. (1999) has investigated the effects of food containing high amounts of reused vegetable oil on plasma lipids and free fatty acids in healthy human subjects (Williams et al. 1999). This study showed that food that are rich in reused vegetable oil caused a significant post-prandial increase in serum triacylglycerides, without altering plasma cholesterol, lipoprotein and free fatty acid levels. Meanwhile, heated PUFA was shown to elevate plasma FFA and triglyceride (Rukkumani, Balasubashini, and Menon 2003).
Based on these studies, it is possible that heated oil-mediated dyslipidemia arise via several mechanisms: (1) through alteration of dietary fatty acids composition in heated vegetable oil, (2) due to lipid peroxidation products of thermally-oxidised oil and (3) due to excess levels of FFA that are associated with heated vegetable oil. Table 1 summarises the fatty acids compositions based on individual lipid profiles, and the effects of heated vegetable oil on these lipids based on meta-analysis and previous studies. Table 2 highlights the possible mechanisms by which dietary fatty acids and lipid peroxidation may regulate apolipoprotein level.
Table 1: Findings on the fatty acids composition and the effects of heated vegetable oil on lipid profiles.
| LDL-C | HDL-C | Total-C | TG | |
| SFA | ↑ | ↓ | ↑ | ↑ |
| cis-PUFA | ↓ | ↑ | ↓ | ↓ |
| cis-MUFA | ↓ | ↑ | ↓ | ↓ |
| trans-MUFA | ↑ | ↓/≈ | NA | NA |
| Heated vegetable oil
Isong et al. Adam et al. Awney et al. El-Deen & Eid Rueda-Clausen et al. |
↑ ↑ ↑ ↑ ≈ |
↑ ↓ ↓ ↓ ≈ |
↑ ↑ ↑ ↑ ≈ |
↑ ↑ NA ↑ ↑ |
Symbols: ↑ increase, ↓ decrease, ≈ no changes. Abbreviations; NA data not available (Source: Mensink et al. 2003; Mensink and Katan 1992; Ooi et al. 2015 (Mensink et al. 2003; Mensink and Katan 1992; Ooi et al. 2015)
Table 2: Mechanisms by which dietary fatty acids and lipid peroxidation regulate apolipoprotein levels.
| Mechanism | References | |
| SFA | ↓LDL receptor
↑ApoA-1 Supress ACAT activity ↓ PPARα |
Mustad et al.1996 (Mustad et al. 1996);
Fernandez and West 2005 (Fernandez and West 2005); Georgiadi & Kersten 2012 (Georgiadi and Kersten 2012) |
| PUFA | ↑LDL receptor
↑/No changes in ApoA-1 ↓ApoB-100 ↑ CYP7A activity ↑ PPARα ↓ SREBP1c ↑ LXR |
Ooi et al. 2015 (Ooi et al. 2015);
Mustad et al. 1996 (Mustad et al. 1996); Georgiadi & Kersten 2012 (Georgiadi and Kersten 2012); Fernandez & West 2005 (Fernandez and West 2005); Shimomura et al. 1999 (Shimomura et al. 1999) |
| trans FA | ↓LDL receptor
↓ApoA-1 ↑Apo B-100 |
Matthan et al. 2004 (Matthan et al. 2004) |
| Lipid peroxidation product | Modify ApoA-1 therefore render HDL dysfunction | Eren et al. 2012 (Eren, Yilmaz, and Aydin 2012);
Brown et al. 2013 (Brown et al. 2013) |
Symbol; ↑ increase, ↓ decrease. Abbreviations: SFA saturated fatty acids; PUFA polyunsaturated fatty acids; FA fatty acids, LDL low density lipoprotein; HDL high density lipoprotein; ApoA apolipoprotein A; ApoB apolipoprotein B; ACAT Acyl coenzyme A:cholesterol acyltransferase; PPARα peroxisome proliferator-activated receptor gamma; CYP7A cholesterol 7alpha-hydroxylase, SREBP1c sterol regulatory element binding protein 1c; LXR liver X receptor
Heated Oil-Induced Dyslipidaemia: Mechanisms, Possibilities and Future Directions
It is indeed challenging to postulate the possible molecular mechanisms through which heated vegetable oil may alter lipid profiles; because of the complexity of the lipid regulation pathway itself. Moreover, to date, most studies on heated oil-induced dyslipidaemia are observational in nature and therefore less mechanistic. However, among the available studies, those focusing on the role of peroxisome proliferator-activated receptor (PPAR) pathway in heated oil-induced dyslipidaemia are perhaps the most anticipated. This is because of its interaction with other lipid-related pathways, such as the nuclear factor kB (NFkB) inflammatory pathway as well as nuclear factor E2-related factor 2 (NRF2) mediated pathway. Indeed, thermally oxidized oil was proven to upregulate the expression of PPARα signalling pathway and its downstream gene Acyl-CoA Oxidase and cytochrome P450 A1 (CYP4A1) gene (Chao et al. 2001). This study also suggested that the increased gene expression further enhanced hepatic fatty acids β-oxidation and reduced liver triglyceride level. Another study by Sulzle et al. 2004 has shown that dietary oxidised fats led to an activation of the liver PPARα gene expression in rats, irrespective of their dietary α-tocopherol concentration supplementation (Sülzle, Hirche, and Eder 2004).
PPARα is a ligand-activated transcription factor which controls a set of genes regulating lipid catabolism (Rueda-Clausen et al. 2007). PPARα is a ligand for both SFA and PUFA with higher affinity for PUFA, thus reiterating the hypolipidaemic effects of dietary PUFA (Georgiadi and Kersten 2012). PPARα upregulate adenosine triphosphate-binding cassette sub-family A member 1 (ABCA1) expression by inducing liver X receptor α (LXRα), promoting cholesterol efflux to apolipoprotein A1 (ApoA1) to form HDL (Zhou et al. 2015). PPARα activation also stimulate the transcription of lipoprotein lipase (LPL) gene. LPL enzymes hydrolyses triglyceride (TG) component of VLDL and chylomicron therefore releasing FFA and reduces plasma TG concentrations. The released free fatty acids are subsequently oxidised via β-oxidation pathway. In fact, PPARα activation also enhanced β-oxidation (Hihi, Michalik, and Wahli 2002). Therefore, PPARα activation may lower both the triglyceride and VLDL levels via enhancing catabolism of TG-rich lipoproteins as well as resulting in a concomitant increase in the uptake and metabolism of the released fatty acids (Schoonjans, Staels, and Auwerx 1996). In contrast to PPARα, sterol regulatory element-binding proteins (SREBP) gene is an important determinant of lipogenesis. In general, SREBPs stimulate the expression of many other genes involved in the synthesis of cholesterol, fatty acids, triglycerides and phospholipids. Dietary PUFA suppress the expression of SREBP especially the isoform SREBP-1 (Georgiadi and Kersten 2012).
As previously mentioned, apart from modulation of gene expression that is involved in lipid metabolism, the concentration of LDL in circulation is determined by the presence of LDL receptors, ApoB availability and the secretion of VLDL (Fernandez and West 2005) which is the precursor of LDL (Izzat, Deshazer, and Loose-Mitchell 2000). Furthermore, the secretion of VLDL is influenced by ApoB availability in the liver as well as the activities of lipid regulatory enzymes such as Acyl coenzyme A:cholesterol acyltransferase (ACAT), 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMGCOA) reductase and cholesterol 7alpha-hydroxylase (CYP7A) (Fernandez and West 2005). ACAT is an intracellular enzyme that catalyzes the formation of cholesteryl esters from cholesterol and long chain fatty acids. The ACAT-derived cholesteryl esters are contained in VLDL remnants and also LDL. In addition, hepatic ACAT activity was significantly correlated with ApoB production whereby the inhibition of ACAT resulted in a decrease in both VLDL and LDL apoB concentrations (Burnett et al. 1999). Consumption of trans fatty acids reduced the catabolism of LDL apoB while enhancing the catabolism of ApoA-1 which was associated with increased total and LDL cholesterol levels while simultaneously reducing HDL concentrations (Matthan et al. 2004). Meanwhile, lipid peroxidation products such as peroxyl radicals, hydroxyl radicals and aldehydes modify ApoA-1 therefore resulting in HDL dysfunction (Eren, Yilmaz, and Aydin 2012). Reactive aldehydes were also reported to cause ApoA1 glycation thereby altering the affinity of phospholipids (Brown et al. 2013).
In addition, accumulation of hepatic free cholesterol is prevented by a decrease in HMG-CoA reductase activity and increases in cholesterol 7α-hydroxylase (CYP7) activity (Izzat, Deshazer, and Loose-Mitchell 2000). Meanwhile, the expression of CYP7 is induced by LXRα. Therefore, PUFA may induce LXRα which further upregulate CYP7, thereby facilitate the conversion of cholesterol to bile acids thus irreversibly eliminate excess cholesterol (Fernandez and West 2005).
It is quite possible that lipid peroxidation and oxidative stress releases more fatty acids from their triacylglycerol anchorage (North, Spector, and Buettner 1994). In such an event, an elevation of free fatty acids after the intake of oxidised oil could therefore affect lipid profile. Indeed, elevated FFA and impaired FFA suppression are associated with hypertrigliceridemia such as typically seen in obesity (Ebbert and Jensen 2013). The role of lipid peroxidation product in PPRAα regulation is not well understood. It was reported that hydroxyl MUFA activate PPARs (Yokoi et al. 2010). In addition other study reported that induction PPARα increased the production of superoxide and hydroxyperoxide human and murine macrophages via stimulation of NADPH oxidase activity (Teissier et al. 2004). Whether this negative effect of PPARα agonists is an adaptive mechanism is not certain. However, it is well established that lipid peroxidation products resulted in the formation of oxidized LDL (Ng et al. 2012). It is important to note that apart from hyperlipidemia per se, the oxidative modification of LDL may be of particular importance in the development of heated vegetable oil induced cardiovascular disease. Oxidised LDL causes oxidative damage to the endothelium, encourage macrophage uptake and foam cells formation and eventually results in atherosclerotic plaque development. Ng et al. 2012 (Ng et al. 2012) demonstrated increased in LOX-1, the scavenger of ox-LDL in heated palm and soy oil-induced hypertensive rats. The rats fed with repeatedly heated sunflower oil had higher TBARS contents in serum and in all lipoproteins (HDL, LDL and VLDL) than fresh sunflower oil. Oxidised HDL was thought as protective mechanism to prevent LDL oxidation. Another important finding in this study is that repeatedly heated sunflower oil added with butylated hydroxytoluene and butylated hydroxyanisole but these did not appear to be completely effective in blocking the peroxidative stress in the treated rats. (Garrido-Polonio et al. 2004).
Antioxidants and its Effect on Blood Lipid Profiles and Oxldl with Heated Oil
To the best of our knowledge, to date, there is currently no available studies which reports on the effects of polyphenol therapy towards heated vegetable oil-induced dyslipidemia. However, a recent animal study reported that feeding with heated vegetable oil for 6 months caused an increase in cardiac tissue free fatty acids (FFA) and triglycerides (TG) levels (Sukalingam et al. 2016a) The cardiac levels of FFA and TG were markedly attenuated following treatment with a polyphenol-rich plant extract known as ADD-X. Meanwhile, many studies have also shown that polyphenols may improve lipid profile in an atherosclerotic animal model. For instance, flavonoids extracted from Stellera chamaejasme L. at the dose of 400 mg/kg led to an increase in serum HDL-C while simultaneously decreasing LDL-C, total cholesterol and triglyceride levels in an animal model which were fed with high-fat diet. This study also suggested that flavonoids caused elevated hepatic mRNA expression of Cholesterol 7α-hydroxylase1 (CYP7A1), and peroxisome proliferator-activated receptor (PPAR)-α (Y. Wang et al. 2015) . In a different animal study, intake of grape polyphenols resulted in significant decrease in plasma triglyceride and VLDL concentration although no effects were observed on plasma total cholesterol concentration (Zern, West, and Fernandez 2003). Similarly, hydroxytryrosol found in olive oil was shown to decrease total and LDL-cholesterol as well as triglycerides while simultaneously increasing HDL levels in hyperlipidemic animal model (Gonzalez-Santiago et al. 2006), however no significant lipid lowering effect was reported in a human trial (Lopez-Huertas and Fonolla 2017). Meanwhile, twelve-month consumption of a polyphenol extract from olives which was conducted in a randomized controlled trial has reported that the olive extract improved serum lipid profiles with significant decrease in total- and LDL-cholesterol in postmenopausal women (Filip et al. 2015).
The cholesterol-lowering effects of other type of antioxidant was described earlier by Qureshi et al 2002. In this study, the cholesterol-lowering effects of palm tocotrienol was reported to be associated with a reduction in HMGCOA-reductase enzymes, which are the rate-limiting enzymes in cholesterol synthesis (Qureshi et al. 2002). Therefore, changes in fatty acids composition may be attributable for the heated oil-induced changes in lipid profile. This finding suggested that antioxidants, particularly polyphenols may help to attenuate lipid-raising effects of heated oil. However, the precise mechanisms through which virgin coconut oil (VCO) and vitamin E may have improved serum lipid profiles in animals were still poorly understood. It is not impossible that the observed effects may involve the role of peroxisome proliferator-activated receptor α (PPARα), or its direct effect on lipid regulating enzymes in the liver including HMGCO-A reductase. Moreover, vitamin E and both flavonoid and non-flavonoid phenolic compounds contribute to the protection of LDL from oxidation (Farbstein, Kozak-Blickstein, and Levy 2010; Peyrol, Riva, and Amiot 2017; Vázquez-Velasco et al. 2011). Table 3 lists several important mechanism of actions of polyphenols, in particular flavonoids and vitamin E on dyslipidaemia in general.
Table 3: The mechanism of actions of antioxidants on dyslipidaemia.
| Antioxidant | Findings | Reference |
| Flavonoids | Reduced hepatic HMGCOA, ACAT, increased LDL receptor, reduced ApoB100 secretion, prevent LDL oxidation | Bocco et al. 2016 (Bocco et al. 2016);
Fukuchi et al. 2008 (Fukuchi et al. 2008); Pal et al. 2003 (Pal et al. 2003); Vazquez-Velasco et al. 2011 (Vázquez-Velasco et al. 2011). |
| Vitamin E | Increased hepatic LDL receptor
Reduced hepatic HMGCoA reductase, increased CYP7A; prevent LDL oxidation. |
Chen & Cheng 2006 (Chen and Cheng 2006);
(Farbstein, Kozak-Blickstein, and Levy 2010) |
Abbreviations: LDL Low density lipoprotein; Apolipoprotein (Apo) B100; CYP7A cholesterol 7alpha-hydroxylase; ACAT Acyl coenzyme A:cholesterol acyltransferase, HMGCOA 3-hydroxy-3-methyl-glutaryl-coenzyme (HMGCO) A
A summary of the possible mechanisms through which heated vegetable oil may induce dyslipidemia via the alteration in fatty acids composition, lipid peroxidation product and/or oxidative stress and excess of FFA is depicted in Figure 1. The possible sites of action at which antioxidants may interfere in the mechanisms are also proposed in the figure.
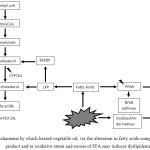 |
Figure 1: Proposed mechanisms by which heated vegetable oil, via the alteration in fatty acids composition, lipid peroxidation product and/or oxidative stress and excess of FFA may induces dyslipidemia. |
Arrow indicate induce. Symbol: ↑ increase; ↓ decrease; possible site of action by which antioxidants may interfere in the pathway. Abbreviations: ACAT, Acyl coenzyme A:cholesterol acyltransferase; CYP7A1, Cholesterol 7 alpha-hydroxylase; SREBP, sterol regulatory element-binding proteins; PPAR, peroxisome proliferator-activated receptors; LXR, liver X receptor
The Effects of Antioxidants and Heated Oil on Cardiovascular Structure
Cardiovascular Remodeling
Studies have reported that prolonged consumption of heated vegetable oil is associated with increased aortic intima-media thickness, intima-media area and circumferential wall tension. Furthermore, it was also proven that hypertension induced by the repeatedly heated vegetable oil simulates a pressure-overload hypertension model, since the increase in blood pressures were gradual and sustainable. Pressure overload may cause increased in circumferential wall tension and intima-media thickness. Despite increment in intima-media thickness, elastic lamina was not significantly affected. These findings further suggested vascular smooth muscle hypertrophy (Siti et al. 2017; Ng et al. 2012; Subermaniam et al. 2015). However, it could not be concluded whether these vascular morphological alterations were directly caused by heated vegetable oil or due to compensatory vascular remodelling. However, many literatures had established the mutual cause-and-effect relationship between hypertension and vascular remodelling (Heagerty et al. 1993; Hayashi and Naiki 2009; Prado and Rossi 2006).
Subermaniam et al. 2015 reported that cardiomyofibre width of left ventricular tissues in heated palm oil-fed rats showed a significant increase in size compared to the control which may suggest left ventricular hypertrophy (Subermaniam et al. 2015). However, another study suggested that cardiac muscle hypertrophy may have not occurred in heated palm-oil treated group (X. F. Leong et al. 2008). This contradictory hypothesis may be due to different method of assessment in which Leong et al. looked into the whole heart weight, while Subermaniam et al. used histomorphometric measurement of left ventricle. Sukalingam et al.2016 on the other hand reported that there was an increase in cardiomyocytes nuclear counts associated with increased cardiomyofibre width of left ventricle in rats fed with heated palm oil; therefore further suggestive of hyperplasia of myocardium (Sukalingam et al. 2016b) . Meanwhile, an experimental model which was used to induce left ventricular hypertrophy by pressure overload in rodents have shown increased levels of superoxide dismutase and glutathione peroxidase activity, and lower malondialdehyde (MDA) levels in the myocardium. There was also associations between lipid peroxidation and left ventricular mass index in a study (Steer et al. 2002) which suggested that lipid peroxidation in plasma may be of importance for growth of the left ventricle. However, pressure overload itself may induce left ventricular hypertrophy as part of compensatory remodelling.
A study has reported that supplementation of flavonoids-rich citrus leaves extract significantly reduced aortic intima-media thickness, intima-media area and circumferential wall tension in five-time-heated palm oil but not in ten-time-heated palm oil group (Siti et al. 2017). This suggests that flavonoids prevent the occurrence of vascular remodeling. This findings were parallel with previous studies which reported that the increased in intima media thickness and myofibre size were significantly prevented by virgin coconut oil supplementation at the dose of 1.42ml/kg orally for 16 weeks (Subermaniam et al. 2015). Sukalingam et al. again demonstrated that polyphenol-rich ADD-X extract was capable to reduce the increment in cardiomyofibre width , which proves the protective effects of ADD-X extract (Sukalingam et al. 2016b).
Atherosclerosis, Cardiovascular Toxicity and Cardiac Injury
Vascular changes such as destruction of intimal layer, increase in sub-endothelial thickness, deposition of collagen and intimal thickening, condensation of cytoplasm and karyopyknosis of endothelial cells, presence of vacuole and collagen in endothelial layer were all observed with diet that contains heated oil. These changes suggest an early atherogenesis process. A study has observed that these vascular changes were severe in the five times-heated palm and soy oil group compared to the once-heated oil group (Adam, Das, and Jaarin 2009; Adam et al. 2009). In this study, it was also found that the detrimental effects of low-estrogenic state on vascular endothelium can be prevented by fresh palm and soy oil supplementation. This effect was lost when the oils were repeatedly heated. The result of this study suggested that vegetable oil contain substances that could prevent blood vessel damage. This could be the high antioxidant content such as vitamin E, which was destroyed with repeated heating (Adam et al. 2007). Another study (Aziz et al. 2012) reported that heated palm oil caused vascular intimal thickening, collagen deposition, condensation of cytoplasm, disruption of internal elastic lamina, as well as the presence of mononuclear cells and vacuolization. However, this structural change was prevented by supplementation of curcumin in a dose of 50mg/ml/kg body weight. This finding suggests that antioxidant curcumin helped to attenuate heated-oil induced vascular changes that predispose to atherosclerosis in post-menopausal rat’s model. Interestingly, Sukalingam et al. 2017 demonstrated high intramyocardial lipid accumulation in cardiac tissues, in the groups that were fed with heated palm oil (unpublished data). Although this research did not concomitantly study lipid accumulation in the vascular wall, it is not impossible that the signs of necrosis observed in the myocardium are partly due to ischemia secondary to atherosclerosis of coronary arteries. Polyphenol-rich ADDX ectract supplementation in heated palm-oil fed group seems to have lesser intramyocardial lipid accumulation compared to palm oil fed-group without ADDX supplementation (unpublished data by Sukalingam et al. 2017).
Leong et al. 2008 reported that 5 times and ten times heated palm oil caused necrosis of cardiac tissue (X. F. Leong et al. 2008). This finding was supported by Subermaniam et al. 2015 which reported that heated palm oil reduced nuclear size which indicated an early sign of pyknosis (Subermaniam et al. 2015). The reduction in nuclear size were significantly prevented by virgin coconut oil supplementation at the dose of 1.42ml/kg orally for 16 weeks. Sukalingam et al. 2016 also reported that heated palm oil caused a reduction in nuclear size but this effects seemed to be attenuated by polyphenol-rich ADD-X supplementation (Sukalingam et al. 2016b).
Lipid peroxidation is the main culprit in oxidised vegetable oil-induced cardiotoxicity (Rouaki et al. 2013). The relationship between cardiotoxicity and lipid peroxidation of cell membrane lipids can be established by looking into the antioxidant system and lipid peroxidation product. Oxidised sunflower oil diets in rats resulted in a reduction in tissue catalase and glutathione peroxidase activities while an increase in lipid peroxidation level was observed. These changes in the heart antioxidant system was associated with the presence of areas of necrosis on the cardiac tissue sections. Interestingly, this study revealed that administration of a moderate dose α-tocopherol (600 mg/kg) restored the antioxidant balance, but that high levels of α-tocopherol (1200 mg/kg) resulted in a pro-oxidant effect which decreased catalase and glutathione peroxidase activities while increasing lipid peroxidation levels (Rouaki et al. 2013). Another study reported rather parallel findings, which was the presence of necrotic cardiac tissues in five-time and ten times-heated palm oil; with the latter showing more severity (Sukalingam et al. 2016b). No significant changes in cardiac histology for control and the experimental group which was fed with polyphenols-rich extract ADD-X, however there was associated reduction in serum thiobarbituric acid reactive substance (TBARS) levels while increased in catalase, reduced glutathione (GSH) and sodium dismutase (SOD) levels were observed. Therefore, this finding suggested that polyphenols-rich antioxidant like ADD-X has cardio-protective effects and is able to prevent heated oil-induced cardiac toxicity. This study also found that heated palm oil consumption increases cardiac LDL and troponin T levels which are often associated with cardiac necrosis in the post-menopausal rat models. Those changes were attenuated by polyphenol-rich ADD-X supplementation in the experiment (Sukalingam et al. 2016a).
In a separate study, pre-treatment with polyphenol-rich lemon grass at a dose of 200 mg/kg in isoproterenol-induced myocardial necrosis decreased the toxic lipid peroxidation events (measured by TBARS levels) in both serum and heart tissues, by increasing the level of enzymatic antioxidants superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione-s-transferase (GST) as well as non-enzymatic antioxidants including reduced glutathione (GSH), vitamin E and vitamin C. In this experiment, the activity of creatinine kinase-MB (CKMB), creatinine kinase (CK), and lactate dehydrogenase (LDH) was observed to be decreased significantly in heart tissues’ homogenate and increased in serum of cardiac-injured rats compared to control group. The cardio-protective effects of lemon grass also was comparable with that of vitamin E (Gayathri et al. 2011). However, care should be taken in interpreting cardiac markers because of their levels are influenced by the timing of myocardial injury as well as the specificity of the type of biomarker.
Conclusion
Heated oil has been proven to be detrimental to the blood pressure, lipid profiles as well as promoting atherosclerosis and cardiac toxicity. These cardiovascular health effects were associated with an increase in lipid peroxidation products and antioxidants enzymes. Supplementation with antioxidants such as polyphenols and vitamin E indeed showed protective effects against the cardiovascular-related parameters. Therefore, the consumption of antioxidant-rich diet or supplementation can aid to attenuate these cardiovascular health effects in animal models. Perhaps more extensive studies need to be conducted to prove their beneficial effects, specifically in human subjects.
Acknowledgments
The authors would like to acknowledge Universiti Kebangsaan Malaysia for the financial support.
Disclosure of Conflict of Interest
None.
References
- Ng CY, Kamisah Y, Faizah O, Jubri Z, Qodriyah HM, Jaarin K. Involvement of Inflammation and Adverse Vascular Remodelling in the Blood Pressure Raising Effect of Repeatedly Heated Palm Oil in Rats. International Journal of Vascular Medicine, 2012; 2012
- Jaarin K, Mustafa MR, Leong XF. The Effects of Heated Vegetable Oils on Blood Pressure in Rats. Clinics, 2011; 66 (12): 2125–32.
CrossRef - Leong XF, Mustafa MR, Das S, Jaarin K. Association of Elevated Blood Pressure and Impaired Vasorelaxation in Experimental Sprague-Dawley Rats Fed with Heated Vegetable Oil. Lipids in Health and Disease, 2010; 9 (1): 66.
CrossRef - Adam SK, Sulaiman NA, Top AG, Jaarin K. Heating reduces vitamin E content in palm and soy Malaysian Journal of Biochemistry and Molecular Biology, 2007; 15 (2): 76–7
- Shuid AN, CM Chew, Mohamed N, Jaarin K, and Soelaiman IN. Repeatedly Heated Frying Oil and High Cholesterol Diet Are Detrimental to the Bone Structure of Ovariectomised Rats. International Journal of Pharmacology, 2007; 3 (2): 160–64.
CrossRef - Jaarin K, Hwa TC, Umar NA, Siti AM, Das S. Enzymatic and Microstructural Changes in the Liver of Experimental Rats Fed with Fatty Diet and Fresh or Heated Soy Oil Concurrently. La Clinica Terapeutica, 2009; 161 (5): 429–33.
- Kamisah Y, Ang SM, Othman F, Nurul-Iman BS, Qodriyah HM. Renoprotective Effect of Virgin Coconut Oil in Heated Palm Oil Diet-Induced Hypertensive Rats. Applied Physiology, Nutrition, and Metabolism, 2016; 41 (10): 1033–38.
CrossRef - de Souza MO, Silva M, Silva ME, de Paula Oliveira R, Pedrosa ML. Diet Supplementation with Acai (Euterpe Oleracea Mart.) Pulp Improves Biomarkers of Oxidative Stress and the Serum Lipid Profile in Rats. Nutrition ,2010; 26 (7): 804–10.
CrossRef - Natsume, M, Baba S. Suppressive Effects of Cacao Polyphenols on the Development of Atherosclerosis in Apolipoprotein E-Deficient Mice. Sub-Cellular Biochemistry, 2013;77: 189–98.
CrossRef - Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, De Caterina R, Carluccio MA. Mediterranean Diet Polyphenols Reduce Inflammatory Angiogenesis through MMP-9 and COX-2 Inhibition in Human Vascular Endothelial Cells: A Potentially Protective Mechanism in Atherosclerotic Vascular Disease and Cancer. Archives of Biochemistry and Biophysics, 2012; 527 (2): 81–89.
CrossRef - Mukai Y, Sato S. Polyphenol-Containing Azuki Bean (Vigna Angularis) Seed Coats Attenuate Vascular Oxidative Stress and Inflammation in Spontaneously Hypertensive Rats. The Journal of Nutritional Biochemistry, 2011; 22 (1): 16–21.
CrossRef - Widmer RJ, Freund MA, Flammer AJ, Sexton J, Lennon R, Romani A, Mulinacci N, Vinceri FF, Lerman LO, Lerman A. Beneficial Effects of Polyphenol-Rich Olive Oil in Patients with Early Atherosclerosis. European Journal of Nutrition, 2013;52 (3): 1223–31.
CrossRef - da Costa CA, de Oliveira PR, de Bem GF, de Cavalho LC, Ognibene DT, da Silva AF, dos Santos Valença S, Pires KM, da Cunha Sousa PJ, de Moura RS, Resende AC. Euterpe Oleracea Mart.-Derived Polyphenols Prevent Endothelial Dysfunction and Vascular Structural Changes in Renovascular Hypertensive Rats: Role of Oxidative Stress. Naunyn-Schmiedeberg’s Archives of Pharmacology, 2012; 385 (12): 1199–1209.
CrossRef - Quiles JL, Ramırez-Tortosa MC, Gómez JA, Huertas JR, Mataix J. Role of Vitamin E and Phenolic Compounds in the Antioxidant Capacity, Measured by ESR, of Virgin Olive, Olive and Sunflower Oils after Frying. Food Chemistry, 2002; 76 (4): 461–68.
CrossRef - Romano, R, N Manzo, L Le Grottaglie, A Fiore, and V Fogliano. Frying Performance of High Oleic Oil Enriched in Biophenols during Discontinuos and Prolonged Thermal Treatment. Food and Nutrition Sciences, 2013;120
CrossRef - Osim, Eme E, Daniel U Owu, and Kevin M Etta. Arterial Pressure and Lipid Profile in Rats Following Chronic Ingestion of Palm Oil Diets. African Journal of Medicine and Medical Sciences, 1996;335-340
- Soriguer F, Rojo-Martínez G, Dobarganes MC, Almeida JM, Esteva I, Beltrán M, De Adana MS, Tinahones F, Gómez-Zumaquero JM, García-Fuentes E, González-Romero S. Hypertension Is Related to the Degradation of Dietary Frying Oils. The American Journal of Clinical Nutrition, 2003;78 (6): 1092–97.
CrossRef - Hamsi MA, Othman F, Das S, Kamisah Y, Thent ZC, Qodriyah HM, Zakaria Z, Emran A, Subermaniam K, Jaarin K. Effect of Consumption of Fresh and Heated Virgin Coconut Oil on the Blood Pressure and Inflammatory Biomarkers: An Experimental Study in Sprague Dawley Rats. Alexandria Journal of Medicine, 2015; 51 (1): 53–63.
CrossRef - Das S, Hamsi MA, Kamisah Y, Qodriyah HM, Othman F, Emran A, Zakaria Z, Jaarin K. Changes in blood pressure, vascular reactivity and inflammatory biomarkers following consumption of heated corn oil. Pakistan Journal of Pharmaceutical sciences. 2017 (in press).
- Leong XF, Najib MN, Das S, Mustafa MR, Jaarin K. Intake of Repeatedly Heated Palm Oil Causes Elevation in Blood Pressure with Impaired Vasorelaxation in Rats. The Tohoku Journal of Experimental Medicine, 2009;219 (1): 71–78.
CrossRef - Leong XF, Jumat S, Rais MM, Kamsiah J. Effect of Repeatedly Heated Palm Olein on Blood Pressure–regulating Enzymes Activity and Lipid Peroxidation in Rats. The Malaysian journal of medical sciences: MJMS, 2012; 19.1
- Jaarin, K, Masbah N, Nordin SH. Heated Cooking Oils and Its Effect on Blood Pressure and Possible Mechanism: A Review. Int J Clin Exp Med ,2016;9 (2): 626–36.
- Marina AM, Che Man YB, Nazimah SA, Amin I. Antioxidant Capacity and Phenolic Acids of Virgin Coconut Oil. International Journal of Food Sciences and Nutrition ,2009;60 (sup2): 114–23.
- Arunima S, Rajamohan T. Virgin Coconut Oil Improves Hepatic Lipid Metabolism in Rats–compared with Copra Oil, Olive Oil and Sunflower Oil. Indian Journal of Experimental Biology, 2012; 50(11)
- Nordin SH, Kamisah Y, Iliyani MI, Mohamed S, Jaarin K. Citrus Leaf Extract Reduces Blood Pressure and Vascular Damage in Repeatedly Heated Palm Oil Diet-Induced Hypertensive Rats. Biomedicine & Pharmacotherapy ,2017. 87: 451–60.
CrossRef - Jin YR, Han XH, Zhang YH, Lee JJ, Lim Y, Chung JH, Yun YP. Antiplatelet Activity of Hesperetin, a Bioflavonoid, Is Mainly Mediated by Inhibition of PLC-γ2 Phosphorylation and Cyclooxygenase-1 Activity. Atherosclerosis, 2007; 194 (1): 144–52.
CrossRef - Sakata K, Hirose Y, Qiao Z, Tanaka T, Mori H. Inhibition of Inducible Isoforms of Cyclooxygenase and Nitric Oxide Synthase by Flavonoid Hesperidin in Mouse Macrophage Cell Line. Cancer Letters,2003; 199 (2): 139–45.
CrossRef - O’Leary KA, de Pascual-Tereasa S, Needs PW, Bao YP, O’Brien NM, Williamson G. Effect of Flavonoids and Vitamin E on Cyclooxygenase-2 (COX-2) Transcription. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2004;551 (1): 245–54.
CrossRef - Yen PL, Chen BH, Yang FL, Lu YF. Effects of Deep-Frying Oil on Blood Pressure and Oxidative Stress in Spontaneously Hypertensive and Normotensive Rats. Nutrition, 2010;26 (3): 331–36.
CrossRef - Beshel FN, Antai AB, Osim EE. Chronic Consumption of Three Forms of Palm Oil Diets Alters Glomerular Filtration Rate and Renal Plasma Flow. Gen Physiol Biophys, 2014; 33: 251–56.
CrossRef - Lee BH, Lai YS, Wu SC. Antioxidation, Angiotensin Converting Enzyme Inhibition Activity, Nattokinase, and Antihypertension of Bacillus Subtilis (Natto)-Fermented Pigeon Pea. Journal of Food and Drug Analysis, 2015; 23 (4): 750–57.
CrossRef - Wang W, Chen W, Yang Y, Liu T, Yang H, Xin Z. New Phenolic Compounds from Coreopsis Tinctoria Nutt. and Their Antioxidant and Angiotensin I-Converting Enzyme Inhibitory Activities. Journal of Agricultural and Food Chemistry, 2014; 63 (1): 200–207.
CrossRef - Oboh G, Bello FO, Ademosun AO, Akinyemi AJ, Adewuni TM. Antioxidant, Hypolipidemic, and Anti-Angiotensin-1-Converting Enzyme Properties of Lemon (Citrus Limon) and Lime (Citrus Aurantifolia) Juices. Comparative Clinical Pathology ,2015;24 (6): 1395–1406.
CrossRef - Nurul-Iman BS, Kamisah Y, Jaarin K, Qodriyah HM. Virgin Coconut Oil Prevents Blood Pressure Elevation and Improves Endothelial Functions in Rats Fed with Repeatedly Heated Palm Oil. Evidence-Based Complementary and Alternative Medicine
CrossRef
- Andrikopoulos NK, Dedoussis GV, Falirea A, Kalogeropoulos N, Hatzinikola HS. Deterioration of Natural Antioxidant Species of Vegetable Edible Oils during the Domestic Deep-Frying and Pan-Frying of Potatoes. International Journal of Food Sciences and Nutrition, 2002;53 (4): 351–63.
CrossRef - Sambanthamurthi R, Tan Y, Sundram K, Abeywardena M, Sambandan TG, Rha C, Sinskey AJ, Subramaniam K, Leow SS, Hayes KC, Wahid MB. Oil Palm Vegetation Liquor: A New Source of Phenolic Bioactives. British Jo urnal of Nutrition, 2011; 106 (11): 1655–63.
CrossRef - Loke, W.M., Proudfoot, J.M., Hodgson, J.M., McKinley, A.J., Hime, N., Magat, M., Stocker, R. and Croft, K.D. Specific Dietary Polyphenols Attenuate Atherosclerosis in Apolipoprotein E–knockout Mice by Alleviating Inflammation and Endothelial Dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology, 2010;30 (4): 749–57.
CrossRef - Gao T, Zhu ZY, Zhou X, Xie ML. Chrysanthemum Morifolium Extract Improves Hypertension-Induced Cardiac Hypertrophy in Rats by Reduction of Blood Pressure and Inhibition of Myocardial Hypoxia Inducible Factor-1alpha Expression. Pharmaceutical Biology, 2016; 54 (12): 2895–2900.
CrossRef - Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, Mazur A. Hesperidin Contributes to the Vascular Protective Effects of Orange Juice: A Randomized Crossover Study in Healthy Volunteers. The American Journal of Clinical Nutrition, 2011; 93 (1): 73–80.
CrossRef - Goszcz K, Deakin SJ, Duthie GG, Stewart D, Leslie SJ, Megson IL. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Frontiers in Cardiovascular Medicine, 2015; 2: 29.
CrossRef - Sukalingam K, Jaarin K, Saad QH, Mohamed S, Othman F. Consumption of ADD-X and Repeatedly Heated Palm Oil on the Blood Pressure and Oxidative Stress Markers in Ovarectmized Rats. International Journal of Pharmacology,2016b ;12 (5): 514–22.
CrossRef - Després JP, Lemieux I, Dagenais GR, Cantin B, Lamarche B. Evaluation and Management of Atherogenic Dyslipidemia: Beyond Low-Density Lipoprotein Cholesterol. Canadian Medical Association Journal, 2001; 165 (10): 1331–33.
- Pal S, Ho N, Santos C, Dubois P, Mamo J, Croft K, Allister E. Red Wine Polyphenolics Increase LDL Receptor Expression and Activity and Suppress the Secretion of ApoB100 from Human HepG2 Cells. The Journal of Nutrition, 2003;133 (3): 700–706.
CrossRef - Mensink RP, Zock PL, Kester AD, Katan MB. Effects of Dietary Fatty Acids and Carbohydrates on the Ratio of Serum Total to HDL Cholesterol and on Serum Lipids and Apolipoproteins: A Meta-Analysis of 60 Controlled Trials. The American Journal of Clinical Nutrition, 2003; 77 (5): 1146–55.
CrossRef - Ebbert JO, Jensen MD. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients, 2013; 5 (2): 498–508.
CrossRef - Adam SK, Soelaiman IN, Umar NA, Mokhtar N, Mohamed N, Jaarin K. Effects of Repeatedly Heated Palm Oil on Serum Lipid Profile, Lipid Peroxidation and Homocysteine Levels in a Post-Menopausal Rat Model. Mcgill J Med ,2008;11 (2): 145–51.
- Awney HA. “he Effects of Bifidobacteria on the Lipid Profile and Oxidative Stress Biomarkers of Male Rats Fed Thermally Oxidized Soybean Oil. Biomarkers, 2011;16 (5): 445–52.
CrossRef - El-Deen NA, Eid M. Efficacy of Curcumin to Reduce Hepatic Damage Induced by Alcohol and Thermally Treated Oil in Rats. Veterinaria Italiana, 2010; 46 (1): 83–92.
- Isong EU, Essien EU, Umoh IB, Ifon ET, Eka OU. Effects of Ingested Thermoxidised Palm Oil on Lipid Distribution in Rat. Nutrition Research, 1996; 16 (5): 773–80.
CrossRef - Rueda-Clausen, C.F., Silva, F.A., Lindarte, M.A., Villa-Roel, C., Gomez, E., Gutierrez, R., Cure-Cure, C. and López-Jaramillo, P. Olive, Soybean and Palm Oils Intake Have a Similar Acute Detrimental Effect over the Endothelial Function in Healthy Young Subjects. Nutrition, Metabolism and Cardiovascular Diseases ,2007; 17 (1): 50–57.
CrossRef - Alireza S, Tan CP, Hamed M, Che Man YB. Effect of Frying Process on Fatty Acid Composition and Iodine Value of Selected Vegetable Oils and Their Blends. International Food Research Journal ,2010;17 (2): 295–302.
- Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired Endothelial Function Following a Meal Rich in Used Cooking Fat. Journal of the American College of Cardiology, 1999; 33 (4): 1050–55.
CrossRef - Rukkumani R, Balasubashini M, Menon VP. Protective Effects of Curcumin and Photo‐irradiated Curcumin on Circulatory Lipids and Lipid Peroxidation Products in Alcohol and Polyunsaturated Fatty Acid‐induced Toxicity. Phytotherapy Research ,2003; 17 (8): 925–29.
CrossRef - Chao PM, Chao CY, Lin FJ, Huang CJ. Oxidized Frying Oil up-Regulates Hepatic Acyl-CoA Oxidase and Cytochrome P450 4 A1 Genes in Rats and Activates PPARα The Journal of Nutrition ,2001; 131 (12): 3166–74.
CrossRef - Sülzle A, Hirche F, Eder K. Thermally Oxidized Dietary Fat Upregulates the Expression of Target Genes of PPARα in Rat Liver. The Journal of Nutrition, 2004; 134 (6): 1375–83.
CrossRef - Georgiadi A, Kersten S. Mechanisms of Gene Regulation by Fatty Acids. Advances in Nutrition: An International Review Journal ,2012; 3 (2): 127–34.
CrossRef - Zhou L, Li C, Gao L, Wang A. High-Density Lipoprotein Synthesis and Metabolism (Review). Molecular Medicine Reports ,2015;12 (3): 4015–21.
CrossRef - Hihi AK, Michalik L, Wahli W. PPARs: Transcriptional Effectors of Fatty Acids and Their Derivatives. Cellular and Molecular Life Sciences, 2002;59 (5): 790–98.
CrossRef - Schoonjans K, Staels B, Auwerx J. Role of the Peroxisome Proliferator-Activated Receptor (PPAR) in Mediating the Effects of Fibrates and Fatty Acids on Gene Expression. Journal of Lipid Research,1996; 37 (5): 907–25.
- Izzat NN, Deshazer ME, Loose-Mitchell DS. New Molecular Targets for Cholesterol-Lowering Therapy. Journal of Pharmacology and Experimental Therapeutics, 2000;293 (2): 315–20.
- Fernandez Ml, West KL. Mechanisms by Which Dietary Fatty Acids Modulate Plasma lipids1. The Journal of Nutrition, 2005; 135 (9): 2075–78.
CrossRef - Burnett JR, Wilcox LJ, Telford DE, Kleinstiver SJ, Barrett PH, Newton RS, Huff MW. Inhibition of ACAT by Avasimibe Decreases Both VLDL and LDL Apolipoprotein B Production in Miniature Pigs. Journal of Lipid Research, 1999; 40 (7): 1317–27.
- Matthan NR, Welty FK, Barrett PH, Harausz C, Dolnikowski GG, Parks JS, Eckel RH, Schaefer EJ, Lichtenstein AH. Dietary Hydrogenated Fat Increases High-Density Lipoprotein apoA-I Catabolism and Decreases Low-Density Lipoprotein apoB-100 Catabolism in Hypercholesterolemic Women. Arteriosclerosis, Thrombosis, and Vascular Biology, 2004; 24 (6): 1092–97.
CrossRef - Eren E, Yilmaz N, Aydin O. High Density Lipoprotein and It’s Dysfunction. The Open Biochemistry Journal, 2012; 6 (1).
CrossRef - Brown BE, Nobecourt E, Zeng J, Jenkins AJ, Rye KA, Davies MJ. Apolipoprotein AI Glycation by Glucose and Reactive Aldehydes Alters Phospholipid Affinity but Not Cholesterol Export from Lipid-Laden Macrophages. PLoS One,2013; 8 (5): e65430.
CrossRef - North JA, Spector AA, Buettner GR. Cell Fatty Acid Composition Affects Free Radical Formation during Lipid Peroxidation. American Journal of Physiology-Cell Physiology 1994; 267 (1): C177–88.
CrossRef - Yokoi H, Mizukami H, Nagatsu A, Tanabe H, Inoue M. Hydroxy Monounsaturated Fatty Acids as Agonists for Peroxisome Proliferator-Activated Receptors. Biological and Pharmaceutical Bulletin, 2010;33 (5): 854–61.
CrossRef - Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, Brandes RP, Shah A, Staels B. Peroxisome Proliferator–activated Receptor α Induces NADPH Oxidase Activity in Macrophages, Leading to the Generation of LDL with PPAR-α Activation Properties. Circulation Research ,2004;95 (12): 1174–82.
CrossRef - Garrido-Polonio C, García-Linares MC, García-Arias MT, López-Varela S, García-Fernández MC, Terpstra AH, Sánchez-Muniz FJ. Thermally Oxidised Sunflower-Seed Oil Increases Liver and Serum Peroxidation and Modifies Lipoprotein Composition in Rats. British Journal of Nutrition, 2004;92 (02): 257–65.
CrossRef - Sukalingam K, Jaarin K, Saad QH, Mohamed S, Othman F. Effect of Rutacea Plant Extract (ADD-X) on Inflammatory Biomarkers, Cardiac LDL, Troponin T and Histological Changes in Ovariectomized Rats Fed with Heated Palm Oil. International Journal of Toxicological and Pharmacological Research, 2016a; 8 (4): 223–31.
- Wang Y, Li JY, Han M, Wang WL, Li YZ. Prevention and Treatment Effect of Total Flavonoids in Stellera Chamaejasme L. on Nonalcoholic Fatty Liver in Rats. Lipids in Health and Disease, 2015;14 (1): 85.
CrossRef - Zern TL, West KL, Fernandez ML. Grape Polyphenols Decrease Plasma Triglycerides and Cholesterol Accumulation in the Aorta of Ovariectomized Guinea Pigs. The Journal of Nutrition, 2003; 133 (7): 2268–72.
CrossRef - Gonzalez-Santiago M, Martín-Bautista E, Carrero JJ, Fonolla J, Baro L, Bartolomé MV, Gil-Loyzaga P, Lopez-Huertas E. One-Month Administration of Hydroxytyrosol, a Phenolic Antioxidant Present in Olive Oil, to Hyperlipemic Rabbits Improves Blood Lipid Profile, Antioxidant Status and Reduces Atherosclerosis Development. Atherosclerosis,2006; 188 (1): 35–42.
CrossRef - Lopez-Huertas E, Fonolla J. Hydroxytyrosol Supplementation Increases Vitamin C Levels in Vivo. A Human Volunteer Trial. Redox Biology, 2017; 11: 384–89.
CrossRef - Filip R, Possemiers S, Heyerick A, Pinheiro I, Raszewski G, Davicco MJ, Coxam V. Twelve-Month Consumption of a Polyphenol Extract from Olive (Olea Europaea) in a Double Blind, Randomized Trial Increases Serum Total Osteocalcin Levels and Improves Serum Lipid Profiles in Postmenopausal Women with Osteopenia. The Journal of Nutrition, Health & Aging, 2015; 19 (1): 77.
CrossRef - Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-Dependent Suppression of Serum Cholesterol by Tocotrienol-Rich Fraction (TRF 25) of Rice Bran in Hypercholesterolemic Humans. Atherosclerosis, 2002;161 (1): 199–207
CrossRef - Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant Vitamins and Their Use in Preventing Cardiovascular Disease. Molecules,2010;15 (11): 8098–8110.
CrossRef - Peyrol J, Riva C, Amiot MJ. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients, 2017; 9 (3): 306.
CrossRef - Vázquez-Velasco M, Díaz LE, Lucas R, Gómez-Martínez S, Bastida S, Marcos A, Sánchez-Muniz FJ. Effects of Hydroxytyrosol-Enriched Sunflower Oil Consumption on CVD Risk Factors. British Journal of Nutrition, 2011; 105 (10): 1448–52.
CrossRef - Subermaniam K, Saad QH, Kamisah Y, Othman F. Effects of Virgin Coconut Oil on the Histomorphometric Parameters in the Aortae and Hearts of Rats Fed with Repeatedly Heated Palm Oil. International Journal of Bioscience, Biochemistry and Bioinformatics, 2015; 5 (2): 120.
CrossRef - Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small Artery Structure in Hypertension. Dual Processes of Remodeling and Growth. Hypertension, 1993; 21 (4): 391–97.
CrossRef - Hayashi K, Naiki T. Adaptation and Remodeling of Vascular Wall; Biomechanical Response to Hypertension. Journal of the Mechanical Behavior of Biomedical Materials, 2009; 2 (1): 3–19.
CrossRef - Prado CM, Rossi MA. Circumferential Wall Tension due to Hypertension Plays a Pivotal Role in Aorta Remodelling. International Journal of Experimental Pathology, 2006;87 (6): 425–36.
CrossRef - Leong XF, Aishah A, Aini UN, Das S, Jaarin K. Heated Palm Oil Causes Rise in Blood Pressure and Cardiac Changes in Heart Muscle in Experimental Rats. Archives of Medical Research, 2008; 39 (6): 567–72.
CrossRef - Steer P, Millgård J, Sarabi DM, Basu S, Vessby B, Kahan T, Edner M, Lind L. Cardiac and Vascular Structure and Function Are Related to Lipid Peroxidation and Metabolism. Lipids, 2002; 37 (3): 231–36.
CrossRef - Adam SK, Das S, Jaarin K. A Detailed Microscopic Study of the Changes in the Aorta of Experimental Model of Postmenopausal Rats Fed with Repeatedly Heated Palm Oil. International Journal of Experimental Pathology, 2009;90 (3): 321–27.
CrossRef - Adam SK, Das S, Othman F, Jaarin K. Fresh Soy Oil Protects against Vascular Changes in an Estrogen-Deficient Rat Model: An Electron Microscopy Study. Clinics, 2009; 64 (11): 1113–19.
CrossRef - Aziz NU, Othman F, Yusof K, Jaarin K, Das S. Effect of Curcumin On the Cardiovascular System of Ovariectomised Experimental Rats Fed with High Cholesterol and Heated Palm Oil Diet. British Journal of Biomedical Science, 2012; 1 (1): 63–73.
CrossRef - Rouaki F, Mazari A, Kanane A, Errahmani MB, Ammouche A. Cardiotoxicity Induced by Dietary Oxidized Sunflower Oil in Rats: Pro-and Antioxidant Effects of α-Tocopherol. J. Vitam. Nutr Res.,2013; 83 (6): 367–76.
CrossRef - Gayathri K, Jayachandran KS, Vasanthi HR, Rajamanickam GV. Cardioprotective Effect of Lemon Grass as Evidenced by Biochemical and Histopathological Changes in Experimentally Induced Cardiotoxicity. Human & Experimental Toxicology ,2011;30 (8): 1073–82.
CrossRef








