Alok K. Pareek*, P. E. Joseph and Daya S. Seth
School of Chemical Sciences, Department of Chemistry, ST. John’s College, Agra- 282 002 (India).
Abstract
3-methyl pyrazolone derivatives have their importance due to variety of industrial applications and wide range of biological activities have been synthesized by two different methods ( conventional heating and microwave irradiation ) and compare these method in terms of yield and time etc. using malon (R) anilic acid hydrazides (1a,1b) with ethyl 2,3-dioxobutyrate 2(RI ) phenyl hydrazono (a1-l1) were reacted in presence of glacial acetic acid to give 4(R I ) phenyl hydrazono - N1-(R) amino malonyl-3-methyl-pyrazol-5-ones and the synthesized compounds was purified by recrystallization from absolute ethanol. The synthesized compounds (2a,2c,2i,2k,3a, 3e,3g,3i) were found to have significant effect against S.aureus and E.coli micro-organism. The structures of the synthesized compounds have been characterized by their elemental analysis, colour, m.p, yield%, spectroscopic (IR, 1H NMR) technique.
Keywords
Pyrazolones; Spectral studies; Biological activity; Microwave; irradiation; Comparison
Download this article as:| Copy the following to cite this article: Pareek A, Joseph P. E, Seth D. S. Synthesis, Spectral Evaluation and Antimicrobial Screening of some N1- substituted-3-methyl- pyrazol-5-ones by 2 ways: A Comparative Study. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Pareek A, Joseph P. E, Seth D. S. Synthesis, Spectral Evaluation and Antimicrobial Screening of some N1- substituted-3-methyl- pyrazol-5-ones by 2 ways: A Comparative Study. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1640 |
Introduction
Pyrazolone have been studied extensively because of their ready accessibility diverse chemical reactivity and variety of industrial appl-ications variously substituted 3-methyl-pyrazol-5-ones can be effectively utilized as fungicidal1, antibacterial2, antiinflammatory3, analgesic4, bact-ericidal5 agents.
Pyrazolone and it’s derivatives have also been possess anticonvulsants6, cardiovascular7, antidepressant8 biological activities. In the last few years there has been a growing interest in the use of microwave irradiation (MWI) for the synthesis of substituted pyrazolone9, spectacular results have been obtained giving clear indication on the potentialities and advantage of microwave-induced organic reaction enhancement (MORE) technique compare to conventional heating method10-12 . Virtually all types of thermally driven reactions can be accelerated by microwave technique. The use of such no conventional reaction conditions reveal several advantages like a shorter reaction time, increased yield, cleaner reaction. Thus microwave assisted synthesis becomes a part of “Green chemistry” 13-14. In view of this and in continuation15-18 of our interest on organic transformation, we now with to report here the comparative study for the synthesis of substituted 3-methyl-pyrazol-5-ones.
Experimental
Material and Methods
All the mentioned melting points were determined in open capillary tubes and are uncorrected. All the used chemicals in the synthesis were of analytical grade and obtained from Sigma-Aldrich. The purity of the synthesized compounds was ascertained by TLC on silica-gel-coated AI Plates (Merck). The IR spectra was taken (in Kbr)on Perkin-Elmer spectrum RX-1 FT-IR spectrophotometer at ST. John’s College Agra, 1H-NMR spectra was measured on Advanced Bruker DRX-300, using solution in DMSO d6, chemical shifts are given in (ppm) and protons signals are indicated as: s=singlet, d=doublet, t= triplet, m=multiplet, physical properties, elemental analysis are furnished in the Table-1 and spectral data are listed in the Table-2, antibacterial activity results are shown in the Table-3.The microwave irradiations were carried out in an IFB domestic microwave oven.
Synthesis of malon (3-Cl-4-OCH3 , 2-OCH3-5-CH3) anilic acid hydrazide (1a, 1b).
To the substituted aniline (R; 0.025 mole), freshly distilled diethyl malonate (0.05 mole) was added in the presence of dimethyl formamide, refluxed the reaction mixture for 45 minutes, ethanol (20 ml) was added, filtered, concentrated over a boiling water-bath, and then (20 ml) of eth-anol was added together with hydrazine hydrate 99%, thus the resulting solid was separated, purified by recrystallization from absolute ethanol.
Synthesis of ethyl 2, 3 – dioxobutyrate -2 – (RI)
phenyl hydrazono (a1-l1).
To the substituted amine (RI ; 0.025 mole) di-azotised it by adding concentrated HCI (8 ml) and distilled water (7 ml) cooled the solution in an ice-bath at 0OC, the cold aqueous solution of Sodium Nitrite (0.025 mole) was added to it, after that the diazotized salt solution was added drop-wise in to the cooled at 0OC solution of Sodium acetate(0.12 mole) and ethyl aceto acetate (0.025 mole) in ethanol (20 ml), thus the solid part was separating out, filtered, washed with cold distilled water, purified by recrystallization from hot ethanol 99%.
General method A(heating) for the synthesis of 4(RI)phenyl hydrazono-N1(R)amino malonyl-3-methyl-pyrazol-5-ones (2a-2l, 3a-3l).
To (1a,1b; 0.001 mole), (a1-l1 ; 0.001 mole) dissolved in absolute ethanol (10 ml) with few drops of glacial acetic acid was used as a catalyst and refluxed the reaction mixture for 4-5 hours, thus the resulting solid was obtained during refluxing, cooled at room temperature, filtered, purified by recrystallization from absolute ethanol.
General method B (microwave irradiation) for the synthesis of 4-(RI)phenyl hydrazono-N1-(R)
amino malonyl-3-methyl-pyrazol-5-ones (2a-2l, 3a-3l).
To substituted malonic acid hydrazide (1a, 1b; 0.001 mole) and ethyl 2,3-dioxobutyrate-2-(RI) phenyl hydrazono (a1-l1; 0.001 mole) dissolved in ethanol (15 ml) and few drops of glacial acetic acid was added in the reaction mixture, were irradiated in microwave oven for 3-6 minutes. Thus the solid part was obtained, purified by recrystallization from hot absolute ethanol.
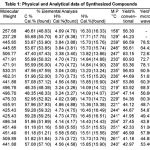 |
Table 1:
|
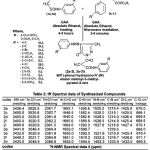 |
Table 2:
|
Antibacterial activity
The synthesized compounds were screened for their antibacterial activity against Staphy-lococcus aureus and Escherichia coli micro organism by filter paper disc diffusion method19-20
was followed by using special Hi-Media sterile disc. The activity was compared with known standard antibacteria drug viz: Streptomycin, the compounds were screened at concentration of 25 μg ml-1 in DMF solution. The zone of inhibition produced by each compounds was measured in mm.
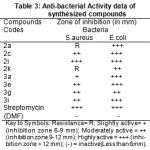 |
Table 3: Anti-bacterial Activity data of synthesized compounds.
|
Results and Discussion
The IR spectra of synthesized compounds have been recorded in the frequency region 4000-500 cm-1 are furnished in the Table-2.
The IR (in Kbr) spectrum of compounds (2a ,2c,2d,2f,2i) shows stretching vibrations in the range 3440.0-3436.4 cm-1 for -NH, stretching vibrations in the range 3020.5-3020.8 cm-1 indicates aromatic -CH, while absorption bands in the range 2361.3-2362.7 cm-1 indicated N=CH stretching vibrations,stretching vibrations between 1640.0-1665.0 cm-1 shown aromatic -C=O, stretching vibrations in the range 1582.4-1595.6 cm-1 reveals C=N, stretching vibrations in the range 1525.2-1530.0 cm-1 indicating the presence of (pyrazolone ring) -N-N, stretching vibrations in the range 1214.9-1215.8 cm-1 indicates C-N, -CH3 group absorption bands are present in the range 1425.0-1427.4 cm-1, mono substitution are present between the range 669.1-670.1 cm-1.
The above observations of IR spectra (in Kbr) are agreed with the assigned structure of the compounds (2a,2c,2d,2f,2i) and other compounds 2b,( 2e,2g,2h,2j-2l ).
The IR spectra of compounds (3a,3e,3f,3h) shows absorption bands in the range 3430.0-3449.3 cm-1 reveals -NH, absorption bands in the range 3020.9-3048.4 indicates aromatic -CH stretching vibrations, absorption bands are present in the range 2361.8-2366.6 cm-1 indicting N=CH stretching vibrations, while absorption bands in the range 1650.0-1652.0 cm-1 reveals aromatic C=O, stretching vibrations in the range 1592.0-1610.2 cm-1 reveals C=N, stret. vibrations in the range 1492.3-1522.5 cm-1 indicates the presence of pyrazolone ring N-N, stretching vibrations are present in the range 1215.9-1233.9 cm-1 due to -C-N, -CH3 group are present in the stretching vibrations between 1425.4-1432.0 cm-1, mono substitution are present in the range of 666.0-671.7 cm-1 .
The above results are sufficient to support the assigned structure of the compounds (3a,3e, 3f,3h ) and other compounds ( 3b-3d,3g,3i.3l ).
The spectral data of 1H-NMR of the compound showed doublet at3.343(CH2), 2.500 (CH3), 3.236 (pyrazolone ring), singlet at 4.337 (-NH), 8.473, 9.100(-CONH), 9.249(-C=O). These results are confirming the structure of compound 3a and other synthesized compounds.
By above results of antibacterial activity most of the substituted pyrazolones showed significant activity. The anti-bacterial activity of compounds 2a,2c,2i,3a,3e,3i highly active against gram negative Escherichia coli micro organism.
Acknowledgements
The author express their sincere thanks to
The Head, Central Drug Research Institute(CDRI) , Lucknow for spectral analysis results (1H-NMR) and Prof. & Head, Department of Chemistry, R.B. S College, Agra providing necessary facilities for antibacterial screening.
References
- M.B.Hogale and B.N.Pawar, J.Ind.Chem.Soc ., 66: 206-207(1989).
- Mohd. Amir, S.M.Hasan and A.Wadood, Orie nt.J.Chem., 18(2): 351-53(2002).
- Anees A.Siddiqui, S.A.Khan and Shibeer Ahmed Bhatt, Orient.J.Chem., 18(2): 375-376 (2002).
- Mohd. Amir and Shikha Kumar, Ind. J. Chem. , 44B, 2532-2537(2005).
- M.S.R.Murthy and E.Venkata Rao, Ind. Drugs , 22(9): 462-64(1985).
- D.Azarifar & M.Shaebanzadeh, Molecules, 7 : 885(2002).
- V.K.Archana, R.C.Shrivastava and A.Kumar, Indian J.Chem., 41B: 1310(2002).
- Palaska, E.M.Aytemir, T.Uzbay and D.Eros., Eur.J.Med.Chem., 36: 639(2001).
- Sonal D.Boob & P.R.Rajpoot, Orient.J.Chem. , 26(3), 879-889(2010).
- S.Caddick, Tetrahedron, 51: 10403(1995).mistry, 1: 43(1999).
- R.S.Verma, Green Chemistry, 1: 43(1999).
- S.A.Galena, Chem.Soc,Rev., 26: 233(1997).
- F.Langa, P.Delacruz and A.Delahazu, Conte-mporary Org. Synth., 373(1997).
- A.K.Bose, M.S.Manhas, M.Ghosh, M.Shah, J. Org.Chem., 56: 6968(1991).
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., 25(1): 203-206(2009).
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., 25(3): 735-738(2009).
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., 25(4): 1059-1063(2009).
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., 25(4): 1087-1091(2009).
- R.Cruickshank, J.P.Duguid, B.P Marion and R.H.A, Medicinal Microbiology, 12th Edn., 2,1 96-202 (1975).
- L.J.Bradshow Ed.,A text book of Microbiology ,W.P.Sounders Co., Philadelphia, New York (1979).







