Omnia E. Kelany1, Howayda E. Khaled2, Amal M. El-Nahla3, Heba M. A. Abdelrazek3 and Mohamed M. Abdel-Daim4
1Department of Clinical Pathology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
2Zoology Department of Faculty, Sciences, Suez University, Egypt.
3Department of Physiology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
4Pharmacology Department of Faculty, Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Corresponding Author E-mail: abdeldaim.m@vet.suez.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/1203
Abstract
This study aimed the assessment of the hepatoprotective effects of dietary soy isoflavones (26%), subclass of phytoestrogens, against hepatic deteriorations induced by hypercaloric diet in cyclic female Wistar rats for 30 days. A total of 32 cyclic female Wistar rats were equally divided into control; (fed on casein based hyper caloric diet) and the other group received soy (26%) fed hyper caloric diet group for 30 days. Weekly food intake and body weight gain, serum lipid profile, leptin, hepatic GSH and MDA were determined. Liver histopathological examination and immunohistochemistry of hepatic ERβ receptors content were also determined. Weekly food intake and body weight gain were significantly (P<0.05) decreased in soy group than control. HDL was significantly (P<0.01) higher in treated group than control during all phases of estrous cycle. Total cholesterol (TC), Triglycerides (TG), and leptin were significantly (P<0.01) decreased in soy treated group than control. Hepatic GSH was significantly increased during proestrus (P<0.01), estrus and metestrus (P<0.05). MDA was significantly reduced in soy fed group during proestrus (P<0.01), estrus, metestrus and diestrus (P<0.05) than control. Histopathology of soy fed group revealed absence of steatosis and fatty infiltrations that were present in control. Hepatic ERβ increased significantly (P<0.01) in soy treated group than control in all phases of estrous cycle. It was concluded that, soy genistein and daidzein have a positive effect toward prevention of adiposity and hepatic oxidative stress caused by hypercaloric diet through ERβ signaling.
Keywords
ERβ; GSH; Isoflavones; MDA leptin;liver;
Download this article as:| Copy the following to cite this article: Kelany O. E, Khaled H. E, El-Nahla A. M, Abdelrazek H. M. A, Abdel-Daim M. M. Hepatoprotective and Metabolic Effects of Dietary Soy Phytoestrogens Against Hyper Caloric Diet in Cyclic Female Albino Rats is Mediated Through Estradiol Receptors Beta. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Kelany O. E, Khaled H. E, El-Nahla A. M, Abdelrazek H. M. A, Abdel-Daim M. M. Hepatoprotective and Metabolic Effects of Dietary Soy Phytoestrogens Against Hyper Caloric Diet in Cyclic Female Albino Rats is Mediated Through Estradiol Receptors Beta. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16353 |
Introduction
Many foods and phytochemicals are considered to have therapeutic or health-promoting properties. Among these compounds; phytoestrogens have received much attention nowadays. They are ubiquitous within the plant kingdom and they have estrogen-like activity.1 They have a phenolic group positioned similarly to that of estrogenic steroids.2 They are categorized into 3 classes; lignans, coumestans and isoflavones.3 Phytoestrogens have been widely consumed by human and animals. Their consumption is attributed to the reduction of numerous chronic diseases; including hormone related cancers, coronary heart disease, osteoporosis, and hypercholesterolemia.4 Phytoestrogens have numerous metabolites that vary greatly in structures but they resemble to the human estrogen; 17ß-estradiol.5 They can bind both estrogen receptors (ERs) alpha (ERα) and beta (ERβ)6 with more preference to ERβ than ERα.7 Phytoestrogens are considered natural selective estrogen receptor modulators (SERMs) as they are associated with both estrogenic and anti-estrogenic activity in the body.8,9 Variation in physiological response to phytoestrogens is tissue specific and dependent on receptors number, extent of protein binding, distribution of receptor subtype and competing estrogen concentrations.5
ERs have been demonstrated by numerous studies in humans and rodents to be essential mediators of the estrogen action on glucose and lipid metabolism.10,11 Moreover, the direct and indirect effects of estrogen on adiposity has been extensively reported as it act by appetite or energy expenditure modulation via controlling release of some hormones such as leptin.11
The oxidative damage is induced by free radicals, highly reactive molecules produced during cell metabolism, and exert their deleterious effects on proteins, lipids and DNA according to the age.12 They cause numerous diseases, such as inflammation, cancer, arteriosclerosis, hypertension and diabetes mellitus.13
Recently, studies have implicated for the importance of ER β as an essential regulator of metabolic diseases. However, the potency of ERβ selective ligands to offset adiposity is not clear. Therefore the aim of this study is to clarify the hepato-protective effect of soy isoflavones (genistein and daidzein) in cyclic female Wistar rats with reference to hepatic ERβ expression. Furthermore, confirm their role on alleviation of hepatic oxidative stress beside the remote effect of soy isoflavones on appetite and leptin secretion.
Material and Methods
Animals
A total of 32 adult cyclic female Wistar rats weighing 190-200 g were purchased from Helwan Lab Animal House, Helwan, Egypt. They were kept in metallic cages 4 rats/ cage. They were kept at natural light rhythm of the day and at room temperature with ad libitum food and water access. Animals were kept for 2 weeks to claim before the start of experiment with casein based diet feeding.
All animals’ sampling and treatment were in accordance with the guideline for animal use and care which approved by the research ethics committee of the Faculty of Veterinary Medicine, Suez Canal University.
Experiment Design
Cyclic female Wistar rats were divided into two groups; Group I: control group, n=16 females, they were fed on a hypercaloric casein based ration. Group II: n=16 females received hypercaloric soy diet containing 26% soybeans. The calorie protein ratio for both control and soy isoflavones diet were the same. Both experimental diets were offered for 30 days.
Dietary Isoflavones Analysis
Dietary Isoflavones, genistein and Daidzein, were determined after their extraction from both experimental diets (control and soy ones) according to Thiagarajan et al.14
Food Intake and Body Weight Gain
Average weekly food intake (gram/ week) and body weight gain (gram/ week) were determined.
Blood and Tissue Sampling
After 30 day treatment, the experimental rats were overnight fasted and weighed. Four animals representing each stage of estrous cycle/ group were sacrificed under the effect of chloroform anaethesia to obtain blood. Separated sera were kept at -20oC for estimation of lipid profile and leptin hormone.
Samples from liver were excised from 4 females/ group for each stage of estrous cycle and divided into two parts. First part was kept at -80oC until preparation of liver homogenate for reduced glutathione (GSH) and malondialdehyde (MDA). The remaining part was immersed in 10% neutral buffered formalin for histopathological and immunohistochemical examination.
Lipid Profile
The serum levels of total cholesterol (TC) (ELITech Diagnostic, France), triglycerides (TG) (ELITech Diagnostic, France) and high-density lipoprotein (HDL) cholesterol (Stanbio Laboratory, USA), were measured according to Tietz.15 Serum low density lipoprotein (LDL) was calculated by Friedwald formula described by Davidson and Rosenson.16
LDL-C = Total Cholesterol- (Triglycerides/5 +HDL-Cholesterol)
Plasma leptin level
Plasma leptin concentrations were assayed using commercial enzyme linked immunoassay rat kit (Code No. 27295, IBL, Japan) according to manufacturer instruction.
Reduced Glutathione Activity
GSH activity in liver homogenate was assayed calorimetrically using BioVision, USA kit at 412 nm absorbance.17
Estimation of Malondialdehyde
The liver homogenate MDA content was calorimetrically assayed at 532 nm absorbance using BioVision, USA commercial kit18
Histopathology
Formalin fixed liver sections were processed using standard histopathological procedures according to Bancroft et al.19
Immunohistochemistry
The livers, were cut into 4 μm sections then mounted on positively charged slides. Primary ERβ antibody (Cat. No. RB- 10658-R7, Thermo Scientific Co., UK) was used for IHC according to Bancroft and Cook.20 For quantitative analysis, the intensity of immunoreactive parts was performed using an image analyzer (Image J program). Seven random fields were selected from each slide of both experimental groups. Within each field, integrated density (IntDen) of eight random parts were analyzed and the mean for them was expressed as field Int Den.21
Statistical Analysis
All data in the current study were expressed as mean ± SE. they were subjected to student T test using GraphPad Prism software (Version 5.01, USA). The criterion of probability for the significance was P> 0.05 and P<0.01 for the high significance.
Results
HPLC analysis of both experimental diets revealed that; control diet had no detectable level of genistein or daidzein, while the soy diet contained 1500µg/g genistein and 600 µg/g daidzein, respectively.
There was no significant difference between control and soy fed groups after the first week in body weight or food intake. Starting from the second week till 4th week, body weight gain and food intake (g /week) showed significant (P<0.05) decrease in group II than group I (Table 1).
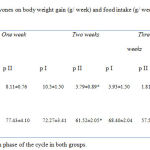 |
Table 1: The effect of dietary soy isoflavones on body weight gain (g/ week) and food intake (g/ week) in cyclic female Wistar rats during 4 weeks.
|
The values of HDL were significantly (P<0.01) higher in soy treated group than control during all phases of estrous cycle. On the other hand, TG and TC were significantly (P<0.01) decreased in group II than group I during all phases of the estrous cycle. LDL revealed non-significant changes between group II and I among all stages of the estrous cycle. Serum leptin levels demonstrated highly significant (P<0.01) decrease in group II during proestrus, estrus, metestrus, and diestrus than group I. The level of hepatic GSH was increased with higher significance (P<0.01) in soy fed group during proestrus phase of the cycle. During estrus and metestrus hepatic GSH showed significant (P<0.05) increase in soy fed group than control meanwhile, during diestrus a non significant increment was observed in soy fed group than control. MDA, denoting Lipid peroxidation, was reduced with higher significance (P<0.01) in hepatic tissue of soy treated group than control during proestrus phase. During estrus, metestrus, and diestrus phases MDA showed a significant (P<0.05) reduction in soy treated livers than control ones (Table 2).
 |
Table 2: Effect of dietary soy isoflavones on serum leptin (ng/ml), HDL (mg / dl), TG (mg / dl), TC (mg / dl), LDL (mg / dl), hepatic GSH (mg/g) and MDA (nmol/g) in cyclic female Wistar rats after 30 days.
|
Liver histopathology demonstrated steatosis and fatty infiltrations in group I while livers of soy fed rats’ showed normal architecture without fat infiltration during all phases of the cycle. ERβ immunostaining showed marked increase in soy fed group during proestrus, estrus, metestrus, and diestrus than control (Figure 1). Moreover, ERβ Integden domenstrated highly significant (P<0.01) increase in soy group than control among all phases of estrus cycle (Figure 2&3).
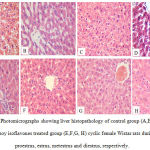 |
Figure 1: Photomicrographs showing liver histopathology of control group (A,B, C,D) and soy isoflavones treated group (E,F,G, H) cyclic female Wistar rats during proestrus, estrus, metestrus and diestrus, respectively.
|
Steatosis and fat infiltration were noted in control group during all phases of the estrous cycle. The soy group revealed no fatty infilterations with normal appearance of hepatic architecture in all phases of the estrous cycle. [hematoxylin and eosin (H&E) stain; original magnification: ×20]
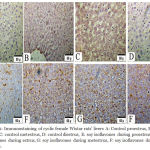 |
Figure 2: Immunostaining of cyclic female Wistar rats’ livers A: Control proestrus, B: control estrus, C: control metestrus, D: control diestrus, E: soy isoflavones during proestrus, F: soy isoflavones during estrus, G: soy isoflavones during metestrus, F: soy isoflavones during diestrus.
|
The Figure demonstrated that ERβ were predominantly localized within the cytoplasm. Soy treatments produced up regulation of ERβ expression in hepatocytes relative to control during all phases of the cycle.X20
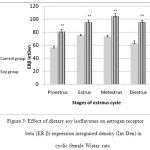 |
Figure 3: Effect of dietary soy isoflavones on estrogen receptor beta (ER β) expression integrated density (Int Den) in cyclic female Wistar rats.
|
Discussion
Current study demonstrated the possible protective effects of soy isoflavones against the risks of adiposity and hepatic oxidative stress in cyclic female Wistar rats. Exposure of mature cyclic female rats to soy isoflavones significantly (P<0.05) reduced food consumption and body weight gain than control ones. Given the physiochemical similarity between soy phytoestrogens and estradiol, it is not surprising that exposure to dietary soy phytoestrogens mimics estrogen’s hormone action as it acts as estradiol. These results are generally consistent with those reported by studies of Tolba22 and Ebaid et al.23 Reduction in food intake may be due to the appetite repressing action of estrogen24 as dietary phytostrogens decrease food intake and hence decrease body weight. The decrease implies that the estrogenic hormone action of soy isoflavones which is beneficial to body fat regulation. Consequently, leptin hormone level that is produced from fat tissue was decreased. Leptin hormone influences hypothalamic neuropeptide Y levels which regulates feeding behaviour.25
Dietary soy isoflavones was shown to have direct effects on lipid metabolism as it decreased TC & TG and increased HDL significantly (P<0.01) in all phases of estrus cycle. These results are consistent with previous records of Uesugi et al.26 and Tolba,22 This could be attributed to the effect of soy isoflavones on hepatic lipid metabolism and adipose tissue that led to decrease in triglycerides.27 This was reflected by decrease in hepatic fatty changes in treated group among all phases of the estrous cycle. These results suggests the hypolipidemic effect of soy isoflavones that are ascribed to their structural similarities to estradiol which acts predominantly via two distinct nuclear ERs, ERα and ERβ, that are defined as ligand-inducible transcription factors.28 Another explanation for the lipid-lowering effect of soy isoflavones; is it might lower cholesterol levels through reduction of intestinal cholesterol absorption with increase in bile acid excretion.29
Soy isoflavones significantly (P<0.01) decreased serum leptin level during proestrus, estrus, metestrus, and diestrus than control. Leptin is a mediator of long-term regulation of energy balance.30 Taking together, the depression in TG and TC as well as the reduced hepatic fatty changes this allowed us to believe the direct influence of soy isoflavones on adipocytes which are the main source of leptin.31 Moreover, soy isoflavones especially genistein inhibit some enzymes in adipocytes that abates their leptin production in spite of unchanged gene expression.32
Soy phytoestrogens increased GSH significantly (P<0.01) during proestrus phase of the cycle and at P<0.05 during estrus and metestrus. On the other hand hepatic MDA was reduced significantly at P<0.01 during proestrus and at P<0.05 during estrus, metestrus, and diestrus phases in soy isoflavones treated livers than control ones. These results were coincide with those of Baeza et al.33 The lipid profile improvements (TC and TG) in soy isoflavones group could be attributed to such effect. Lipid profile alterations is a causal factor for reduction in lipid peroxidation34 and oxidative stress35 that resulted from increase in reactive oxygen species production and reduction in antioxidant enzymes. Hepatic steatosis that shown in group I leaded to an increase in lipid peroxidation in hepatocytes, which, in turn, activates hepatic stellate cells (HSCs). The HSCs are thought to be the primary target cells for inflammatory and oxidative stimuli, and to produce the components of extracellular matrix.36 Moreover, soy isoflavones as SERMs could exert positive effects on liver functions37 as they possess anti-oxidant properties38 thus increasing the hepatic expression of ERβ. Finally, soy isoflavones significantly (P<0.05) increased hepatic ERβ that could modulate energy homeostasis, oxidative stress and lipid peroxidation. However this point needs further investigations and confirmation.
Conclusion
Soy isoflavones improved serum lipid profile, inhibited leptin, modulate hepatic oxidative stress and lipid peroxidation, reduced accumulation of fat in the liver and increased hepatic ERβ expression. Collectively, these results suggest that soy isoflavones can represent a new class of ERβ ligand to prevent/treat adiposity and hepatic oxidative stress.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
This work is fully funded by researchers
References
- Murkies A. L., Wilcox G., Davis S. R. CLINICAL REVIEW 92 Phytoestrogens. Journal of Clinical Endocrinology and Metabolism. 1998;83(2):297-303.
- Setchell K. D., Borriello S. P., Hulme P., Kirk D. N., Axelson M. Nonsteroidal estrogens of dietary origin possible roles in hormone-dependent disease. The American Journal of Clinical Nutrition. 1984;40(3):569-578.
CrossRef - Cederroth C. R., Soy S. N. phytoestrogens and metabolism: A review. Molecular and Cellular Endocrinology. 2009;304(1–2):30-42.
CrossRef - Stark A., Madar Z. Phytoestrogens: A Review of Recent Findings. In.Journal of Pediatric Endocrinology and Metabolism. 2002;561.
CrossRef - Cassidy A., Hanley B., Lamuela‐Raventos R. M. Isoflavones, lignans and stilbenes–origins, metabolism and potential importance to human health. Journal of the Science of Food and Agriculture. 2000;80(7):1044-1062.
CrossRef - Tham D. M., Gardner C. D., Haskell W. L. Potential Health Benefits of Dietary Phytoestrogens: A Review of the Clinical, Epidemiological, and Mechanistic Evidence 1. The Journal of Clinical Endocrinology & Metabolism. 1998;83(7):2223-2235.
CrossRef - Oseni T., Patel R., Pyle J., Jordan V. C. Selective estrogen receptor modulators and phytoestrogens. Planta medica. 2008;74(13):1656.
CrossRef - Setchell K. D., Brown N. M., Desai P., Zimmer-Nechemias L., Wolfe B. E., Brashear W. T., Kirschner A. S., Cassidy A., Heubi J. E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. The Journal of nutrition. 2001;131(4):1362S-1375S.
CrossRef - Abdel-Daim M., Funasaka Y., Komoto M., Nakagawa Y., Yanagita E., Nishigori C. Pharmacogenomics of metabotropic glutamate receptor subtype 1 and in vivo malignant melanoma formation. J Dermatol. 2010;37(7):635-46.
CrossRef - Mauvais-Jarvis F., Clegg D. J., Hevener A. L. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine reviews. 2013;34(3):309-338.
CrossRef - Cooke P. S., Naaz A. Role of estrogens in adipocyte development and function. Experimental biology and medicine. 2004;229(11):1127-1135.
CrossRef - Sohal R. S., Mockett R. J., Orr W. C. Mechanisms of aging an appraisal of the oxidative stress hypothesis 1, 2. Free Radical Biology and Medicine. 2002;33(5):575-586.
CrossRef - Avcı A., Atlı T., Ergüder İ. B., Varlı M., Devrim E., Turgay S. A. M., Durak İ. Effects of apple consumption on plasma and erythrocyte antioxidant parameters in elderly subjects. Experimental aging research. 2007;33(4):429-437.
CrossRef - Thiagarajan D. G., Bennink M. R., Bourquin L. D., Kavas F. A. Prevention of precancerous colonic lesions in rats by soy flakes, soy flour, genistein and calcium. Am J Clin Nutr. 1998;68(6):1394s-1399s.
CrossRef - Tietz N. W. Clinical Guide to Laboratory Tests. Philadelphia, USA W.B. Saunders Company. 1990.
- Davidson M. H., Rosenson R. S. Novel targets that affect high-density lipoprotein metabolism the next frontier. The American journal of cardiology. 2009;104(10):52E-57E.
CrossRef - Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione applications to mammalian blood and other tissues. Analytical biochemistry. 1969;27(3):502-522.
CrossRef - Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351-358.
CrossRef - Bancroft J., Stevens A., Turner D. Theory and practice of histological techniques 4th Ed Churchill Living Stone, New York Edinburgh. Madrid, San francisco. 1996.
- Bancroft J. D., Cook H. C., Bancroft J. D., Cook H. C., editors. Immunohisto chemistry In: Immuno histochemistry manual of histological techniques and their diagnostic applications. New York: W.B. Saunders Company. 1994;263-325.
- Elgawisha R. A. R., Rahman H. G. A., Abdelrazek H. M. A. Green tea extract attenuates CCl4-induced hepatic injury in malehamsters via inhibition of lipid peroxidation and p 53-mediated apoptosis. Toxicology Report. 2015;2:1149–1156.
CrossRef - Tolba EA-EHT. Dietary phytoestrogens reduce the leptin level in ovariectomized female rats. International Journal of Chemical, Environmental & Biological Sciences. 2013;1(3):496-500.
- Ebaid H. M., Elgawish R. A. R., Abdelrazek H. M., Gaffer G., Tag H. M. Prenatal Exposure to Soy Isoflavones Altered the Immunological Parameters in Female Rats. International journal of toxicology. 2016:1091581815625595.
CrossRef - Wade G. N. Some effects of ovarian hormones on food intake and body weight in female rats. Journal of comparative and physiological psychology. 1975;88(1):183.
CrossRef - Naaz A., Yellayi S., Zakroczymski M. A., Bunick D., Doerge D. R., Lubahn D. B., Helferich W. G., Cooke P. S. The soy is of lavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144(8):3315-3320.
CrossRef - Uesugi T., Fukui Y., Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: a four-week study. Journal of the American College of Nutrition. 2002;21(2):97-102.
CrossRef - Nogowski L., Maćkowiak P., Kandulska K., Szkudelski T., Nowak K. W. Genistein-induced changes in lipid metabolism of ovariectomized rats. Annals of nutrition and metabolism. 1998;42(6):360-366.
CrossRef - Rosen E. D., Walkey C. J., Puigserver P., Spiegelman B. M. Transcriptional regulation of adipogenesis. Genes & development. 2000;14(11):1293-1307.
- Greaves K. A., Wilson M. D., Rudel L. L., Williams J. K., Wagner J. D. Consumption of soy protein reduces cholesterol absorption compared to casein protein alone or supplemented with an isoflavone extract or conjugated equine estrogen in ovariectomized cynomolgus monkeys. The Journal of nutrition. 2000;130(4):820-826.
CrossRef - Klok M., Jakobsdottir S., Drent M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans a review. Obesity reviews. 2007;8(1):21-34.
CrossRef - Szkudelski T., Nogowski L., Pruszyńska-Oszmałek E., Kaczmarek P., Szkudelska K. Genistein restricts leptin secretion from rat adipocytes. The Journal of Steroid Biochemistry and molecular biology. 2005;96(3–4):301-307.
CrossRef - Bradley R. L., Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes. 1999;48(2):272-278.
CrossRef - Baeza I., Fdez-Tresguerres J., Ariznavarreta C., De la Fuente M. Effects of growth hormone, melatonin, oestrogens and phytoestrogens on the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio and lipid peroxidation in aged ovariectomized rats. Biogerontology. 2010;11(6):687-701.
CrossRef - Makni M., Fetoui H., Gargouri N., Garoui E. M., Jaber H., Makni J., Boudawara T., Zeghal N. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in ω-3 and ω-6 fatty acids in hypercholesterolemic rats. Food and Chemical Toxicology. 2008;46(12):3714-3720.
CrossRef - Tsimikas S., Miller Y. I. Oxidative modification of lipoproteins: mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Current pharmaceutical design. 2011;17(1):27-37.
CrossRef - Shimizu I., Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatology Research. 2007;37(4):239-247.
CrossRef - Hamden K., Silandre D., Delalande C., ElFeki A., Carreau S. Protective effects of estrogens and caloric restriction during aging on various rat testis parameters. Asian journal of andrology. 2008;10(6):837-845.
CrossRef - Borrás C., Gambini J., Gómez‐Cabrera M. C., Sastre J., Pallardó F. V., Mann G. E., Viña J. 17β‐Oestradiol up‐regulates longevity‐related, antioxidant enzyme expression via the ERK1 and ERK2 [MAPK]/NFκB cascade. Aging cell. 2005;4(3):113-118.
CrossRef








