Maysaa Jalal Majeed
Medicine College -Baghdad University, Baghdad-Iraq.
Correspondent Author E-Mail: dr.alkarkhi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1222
Abstract
Betatrophin, is a new bioactive peptide of 198 amino acids, also known as ANGPTL8, is coded by the Gm6484 gene in mice liver and fat, betatrophinwhich recently found to promote a dramatic proliferation of pancreatic βcells in a S961 induced insulin resistance mouse model it is expressed in the, white and brown adipose tissue of mice. In humans, betatrophin is expressed in the liver. Evaluation of betatrophin as a treatment used for diabetes as an alternate treatment to insulin was studies in mice with type 1 diabetes and comparing the result with control group this study conclude that betatrophin lower blood sugar which lower necessity of depending the patient on insulin.
Keywords
Betatrophin; bioactive peptide; insulin; immunosorbent
Download this article as:| Copy the following to cite this article: Majeed M. J. Impress of Betatrophin in the Treatment of Mice With Diabetes Type 1. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Majeed M. J. Impress of Betatrophin in the Treatment of Mice With Diabetes Type 1. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16099 |
Introduction
Pancreatic-cells are impeccable sensors of bloodglucose levels and secrete just the exact quantityof insulin into the bloodstream to systemicallyadjust glucose and energy homeostasis. Increasing evidence indicates thatdecreased functional beta-cell mass is the hallmarkof Type 2 and type diabetes mellitus (T2DM and T1DM ).1,2
Betatrophin,a new bioactive peptide of 198 amino acids, also known as TD26, RIFL, Lipasin and ANGPTL8, is encoded by the Gm6484 gene in mice liver and fat. In humans, however, betatrophin isprimarily expressed in the liver and encoded by theC19orf80 gene.3,4 Recently, expression of betatrophin is found topromote a dramatic proliferation of pancreaticcells in a S961 induced insulin resistance mousemodel. Moreover, transient expression of beta-trophin in mouse liver significantly and specificallypromotes pancreatic cell proliferation, expands cell mass, and improves glucose tolerance; itopens up the possibility of future betatrophin treatment.5-7
All of mentioned studies were discussed effect of betatrophin in type 2 subjects but the current study creates a hope treatment for diabetes type1.In current study was made on groups of mice, control and patient groups with diabetes type 1 (T1DM) and study how betatrophin can lower the blood sugar.
Material and Methods
This prospective experimental study was based on 46 mice with 1.5-2.0 months-old, the study was carried out at animal house, College of Medicine-University of Baghdad. Medicine, Pharmacology Colleges -University of Baghdad were provide these mice. The animal protocols were approved by the Use and Care of Experimental Animals Committee of the Jichi Medical University Guide for Laboratory Animals.8
Study Design
Thirty six mice were induced type1 diabetes by subcutaneously injection of a single dose of alloxan 0.1mg/g, then the whole mice forty six mice were divide into three groups:
Group 1
Involve 12 DMI mice, after a day from diabetes inducible, they were treated with 0.75 IU insulin/g body weight.(prepare 0.25 IU insulin solution by diluting insulin in sterile saline (and sterile tubes); mix by overtaxing. Dispense the required volume of insulin solution for each mouse into separate 1.5 ml tubes (Volume is calculated as follows: 0.75 IU insulin/g BW). Vol (μl) = 3 x BW). Two of them were dead.
Group 2
Involve 15 DMI mice, after a day from diabetes inducible, they were treated with 0.2 mg betatrophin/g body weight. Six of them were dead.
Group 3
Involve 9 DMI mice, consider as a pathological control for the above group.
Group 4
Involve10 mice represented control non-diabetic mice.
All mice were no significant difference in their body weigh
Methods
Serum glucose was measured by colorimetric methods, Plasma mice C-peptide concentration were measured using an enzyme-linked immunosorbent assay. Fasting blood glucose was measured by using colorimetric methods, these measurements was done in the Teaching laboratories of Baghdad Medicine Hospital.
Subcutaneous injection of prepared Protamine zinc insulin (PZI; a product comprising 90% beef insulin and 10% pork insulin) and lyophilized recombinant betatrophin obtained from phoenix pharmaceuticals.inc
Results
Table 1 shed light on the difference of serum fasting glucose and serum C-peptide among the four mice groups before treatment, which were selected at the age 1.5 -2 months -old.
Table 1: Descriptive characteristics of mice sample (mean ± SD) before treatment
| Parameters | Mice groups | No. | MeanSD | Comparison of significant ANOVA, LSD(f-test) | |
|
F.S.G (mg/dl) |
Inducible type1 diabetic(T1DM) mice before insulin treatment (A) |
12 |
250 25.8 |
0.001HS |
A VS B
NS |
| Inducible type1 diabetic(T1DM) mice before betatrophin treatment (B) |
15 |
253 26.1 | A VS C
NS |
||
| pathological control- Inducible type1 diabetic (c) | 9 | 248 25.5 | B VS C
NS |
||
| Control non-diabetic mice group (D) |
10 |
85 11.5 | A,B,C VS D
0.001 HS |
||
|
S.c-peptide (ng/ml) |
Inducible type1 diabetic(T1DM) mice before insulin treatment (A) |
12 |
2.1 0.9 |
0.001HS |
A VS B
NS |
| Inducible type1 diabetic(T1DM) mice before betatrophin treatment (B) |
15 |
1.9 0.5 | A VS C
NS |
||
| pathological control- Inducible type1 diabetic (c) | 9 | 2.2 0.7 | B VS C
NS |
||
| Control non-diabetic mice group ( D) | 10 | 5.3 1.2 | A,B,C VS D
0.001 HS |
||
The mean values of F.B.G. of the three inducible type1 diabetic(T1DM) mice groups didn’t significantly differ (250±25.8mg/dl,253±26.1mg/dl,248±25.5 mg/dl)but they were significantly higher than control –nondiabetic mice group group ( 85 ± 11.5 mg/dl).C-peptide of the two inducible type1 diabetic(T1DM) mice groups also didn’t significantly differ( 2.1±0.9 ng/ml, 1.1±0.5 ng/ml),and had significantly lower level when compared with control mice group ( 5.3±1.2 ng/ml ).
In addition, a negative correlation was found between serum betatrophin levels and F.S.G in mice before treatment (r = – 0.45; P < 0.01).
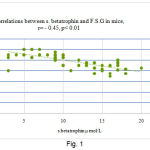 |
Figure 1
|
In the whole mice before treatment, the circulating levels of serum betatrophin positively correlated with c-peptide (r = 0.41; P <0.01).
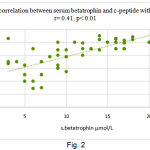 |
Figure 2
|
Management of glucose level by insulin and betatrophin injection clarify by table2
Table 2: Mean distribution of F.S.G and c-peptide in mice groups that treated with insulin injection, betatrophin and their control
| parameters | Mice groups | No. | MeanSD | Comparison of significant ANOVA, LSD(f-test) | |
|
F.S.G (mg/dl) |
Inducible type1 diabetic(T1DM) mice with insulin inj. treatment (A) |
12 |
90.0 10.8 |
0.01 HS |
A ,D VS B NS |
| Inducible type1 diabetic(T1DM) mice with betatrophin inj. treatment (B) |
15 |
75.0 12.9 |
|||
| pathological control- Inducible type1 diabetic (c) | 9 | 250 25.9 |
A,B,D VS C HS |
||
| Control non-diabetic mice group (D) |
10 |
85.0 17.0 | |||
|
S.c-peptide (ng/ml) |
Inducible type1 diabetic(T1DM) mice with insulin inj. treatment (A) |
12 |
2.3 0.85 |
0.001 HS |
A VS B
0.01 S |
| Inducible type1 diabetic(T1DM) mice with betatrophin inj. treatment (B) |
15 |
3.3 2.2 | A VS C,D
0.001 HS |
||
| pathological control- Inducible type1 diabetic (c) |
9 |
1.9 0.9 | B VS C,D
0.001 HS |
||
| Control non-diabetic mice group ( D) | 10 | 5.3 1.2 | C VS D
0.001HS |
||
The data in table 2 revealed that inducible type1 diabetic (T1DM) mice with betatrophin inj. treatment had highest significant mean of c-peptide (3.3±2.2ng/ml) among other mice group, inducible type1 diabetic(T1DM) mice with insulin inj. Treatment (2.0 ± 0.85 ng/ml), pathological control- Inducible type1 diabetic and Control non-diabetic mice group .but both groups had significant lower mean than control non-diabetic mice (5.3 ± 1.2 ng/ml).
Fasting glucose level showed the highest significant in level pathological control- Inducible type1 diabetic among groups p >0.001.
Discussion
Table 1 and graph 1 represent the hyperglycemia of mice after beta-cells has been killed to develop typ1 diabetes (T1DM) using alloxan (toxic glucose analogue, which selectively destroys insulin-producing cells in the pancreas (that is beta cells), alloxan has a high affinity to SH-containing cellular compounds which essential for insulin secretion and, as a result, decrease glutathione content. When a type 1 or type 2 diabetic starts to lose beta cells, the body cannot adequately refill them, and thus diabetics become hyperglycemic as their shrink stores of beta cells which become unable to produce enough insulin.9-11
The results in Table2 and Graph 2 revealed to the significant improve of glucose level in (T1DM) mice using betatrophin therapy at the same time it was found a notable increase in c-peptide of this group,this decline in the level of glucose in group that injected with betatrophin reflect the possible ability of betatrophin in glucose hemostats management which related to its role in controlling the proliferation of the Pancreatic β Cells, hereby Betatrophin can be consider as a bright hope for diabetes control. It was found also from Table 2 the support that a notable increment in c-peptide level of mice group treated with betatrophin while mice group treated with insulin keep the same level of c-peptide.12-13
However there is limited supply of beta cells,17-18 there is much to be gained from having a rebuilding source of new beta cells, but in recent studies scientists have found a hoping of new way to stimulate the body’s own beta cells to replicate.Further experiments showed that 8-week-old mice injected with betatrophin showed an average 17-fold rise in the replication of their β cells.14-15
Conclusion
Data suggested that betatrophin is lower blood sugar and might be hold a new treatment for the dependence of diabetic patients on insulin injection treatment, but this study need for further work to say that betatrophin can replace the insulin treatment.
References
- Park Y. P and Melton J. S. Betatrophin a hormonethat controls pancreatic beta cell proliferation. Cell. 2013;153:747-58.
- Jiao Y., Lay J. L., Yu M., Naji A and Kaestner K. H. Elevated mousehepatic betatrophin expression does not increase humanbeta-cell replication in the transplant setting. Diabetes. 2014;63:1283-8.
- Xing R. G., Xiao L and others. ANGPTL8/betatrophin alleviates insulin resistance via the Akt-GSK3β or Akt-FoxO1 pathway in Hep G2 cells. CellRes. 2016;345:158–167.
- Robert L., Fatjon L and others. The Arg 59 Trp variant in ANGPTL 8 (betatrophin) is associated with total and HDL-cholesterol in American Indians and Mexican Americans and differentially affects cleavage of ANGPTL 3. Molecu. Gen. and Metab. 2016;118:128–137.
- Hale T., Ayhan A., Ahmet A. et al. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diab. Res.clin. pract. 2016;114:37-42.
- Nasser M., Shakilur R. et al. Circulating betatrophin in healthy control and type 2 diabetic subjects and its association with metabolic parameters. Journal of Diab. Comp. 201630:1321–1325.
- Zhang R. Lipasin a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem.Biophys Res Comu. 2016;424:786-792.
- Bailey M. R., Rich B. A., Bennett B. T. Crisis planning to manage risks posed by animal rights extremists. ILAR J. 2010;51:138-148.
- Rankin M. M., Wilbur C. J., Rak K. et al. Beta cells are not generated in pancreatic duct ligation induced injury in adult mice. Diabetes. 2013;62:1634–1645.
- Darambazar G., Nakata M., Okada T., Wang L., Li E., et al. Paraventricular NUCB2/nesfatin-1 is directlytargeted by leptin and mediates its anorexigenic effect. Biochem.Biophys Res Commun. 2015;456:913-918.
- Danilova I. G., Sarapultsev et al. Morphological Restructuring of Myocardium during the Early Phase of Experimental Diabetes Mellitus. Anat Rec. 2014. doi: 10.1002/ar.23052.
- Rigalli A., Di Loreto, Veronica E.,Sun D.,Poureslami H. R. Experimental Surgical Models in the Laboratory Rat, Published by CRC Press ,chapter 9. 2009;43.
- Jonas A. R and Ole D. M. The long awaited circulating factor from the liver promoting β-cell replication. 9:11-12.
- Leszek S. z. Role of immune system in type 1 diabetes mellitus pathogenesis. Inter. Immunopharma. 2014;22:182–191.
- Amnah S., Jamil A. et al. Structural characterization of ANGPTL8 (betatrophin) with its interacting partner lipoprotein lipase. Compu. Bio.Chem. 2016;61:210–220.








