Manuscript accepted on :June 17, 2017
Published online on: --
Plagiarism Check: Yes
Fatmawaty1, Rosmalena1, Amanda Amalia1, Innayah Syafitri1 and Vivitri D. Prasasty2
1Department of Medical Chemistry, Faculty of Medicine, University of Indonesia, Jalan Salemba Raya 6, Jakarta 10430, Indonesia.
2Faculty of Biotechnology, Atma Jaya Catholic University of Indonesia Jalan Jenderal Sudirman 51, Jakarta 12930, Indonesia.
Corresponding Author E-mail: vivitri.dewi@atmajaya.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1206
Abstract
Plasmodium resistance of antimalarial drugs has been a major public health threat worldwide. New treatments are urgently required to harness and eradicate malaria in developing countries. One of the ways to overcome this issue is to develop new therapy from medicinal plants. The purpose of this study is to determine antiplasmodium activities from bark extract of Delonix regia and leaf extract of Carica papaya L., also to compare each ratio combination to find the most effective bioactivity. The experimental design was examined in eleven groups of mice, consisted of the positive control, negative control, Delonix regia bark extract with three different doses (4.80, 9.75, and 15.5 mg/g body weight/body weight of mice), Carica papaya leaves extract with three different doses (4.80, 9.75 mg/g, 15.5 mg/g body weight/BW of mice), and three combinations of Delonix regia and Carica papaya L. with ratio of 1:1, 1:3, and 3:1, respectively. The extract treatments were given daily for five days and on each day the parasite density were evaluated. Parasitemia examination was performed on before and after the treatment by performing blood smear preparation stained with Giemsa stain. The results showed that the plant extracts increased a drastically inhibition in combination of Delonix regia and Carica Papaya L with ratio of 1:1 by showing its efficacy in rectifying immune conditions in Plasmodium berghei-infected mice. Meanwhile, each single dose of Delonix regia and Carica papaya L in 9.75 mg/g showed the highest inhibition rate compared in 4.80 and 15.5 mg/g, respectively. Histological examination of liver tissues of treated and untreated mice further supports the potential antimalaria of this plant. This examination validates the traditional use of this plant for the treatment of malaria.
Keywords
Carica papaya L; Delonix regia; Plasmodium berghei; percent inhibition; Swiss-webster mice
Download this article as:| Copy the following to cite this article: Fatmawaty F, Rosmalena R, Amalia A, Syafitri I, Prasasty V. D. Antimalarial Effect of Flamboyant (Delonix Regia) Bark and Papaya (Carica papaya L.) Leaf Ethanolic Extracts Against Plasmodium Berghei in Mice. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Fatmawaty F, Rosmalena R, Amalia A, Syafitri I, Prasasty V. D. Antimalarial Effect of Flamboyant (Delonix Regia) Bark and Papaya (Carica papaya L.) Leaf Ethanolic Extracts Against Plasmodium Berghei in Mice. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=15803 |
Introduction
Malaria represents a major health issue worldwide, including Eastern Indonesia. There are 433,326 clinical cases out of 232 million Indonesian population in 2005 1-3 and malaria outbreak occurred in some areas causing 87 deaths from 18,812 population in 62 villages in Indonesia in 2005 4-7. The main drive for this deadly infectious disease was the development of multi-resistance of parasite populations, Plasmodium sp. against antimalaria aminoquinolines, including chloroquine, amodiaquine and mefloquine. Plasmodium is a parasite that causes malaria. The typical symptoms of malaria are easily Plasmodium is transmitted from one person to another through the intermediary of the Anopheles mosquito. Some efforts to eradicate malaria are still developed so far, such as making a combination therapies of malaria drugs as a worldwide replacement most Plasmodium falciparum isolates in endemic areas which have found multi-resistance to the aminoquinolines. Artemisinin derivatives became promising drug alternatives in combination with aminoquinolines and other drugs to treat the infection caused by multidrug resistant strains 8, 9. However, artemisinin has been found no longer powerful to eradicate malaria due to its resistance has been reported 10-13, making the development of new antimalarial drugs obtained from natural sources are important.
Populations in Indonesia are common in using traditional medicine due to the cultural acceptability of healers, the relatively low cost of traditional medicine and intention of less toxic remedies. The studies conducted on the traditional medicinal plants in Indonesia are extensive developed to obtain more potent medicinal plants for cancers and infectious diseases. Although recently there are efforts to identify and screen antimalarial herbs used in the ethnomedicine practice of the country, the studies done are very limited and not fully explored. Thus, pharmacological screening of medicinal plants as antimalaria is highly needed. Medicinal plants have been focused for the search of new antimalaria drugs in various parts of the world and the present global situation indicates a recent rising from malaria pecularity, due to the resistance of malaria parasites to pledge antimalaria drugs 14, 15. Thus, it is needed to broaden research in the development of new, cheap and effective antimalaria drugs from medicinal plants. Indonesia is a tropical country which is rich of biodiversities of natural medicinal compounds to heal illnesses.
Delonix regia is a flowering plant in the pea family found in tropical areas and its leaves are used traditionally to treat diseases in folk medicine including antimalaria, especially in the area of East Nusa Tenggara, as by drinking water decoction of Delonix regia leaves. Delonix regia extract was reported to have a wide range of bioactivities. The plant has shown a promising antioxidant 16, antimicrobial 16, 17, antidiabetic 18, and anti-inflammatory effects 19. Delonix regia leaf extract was also reported became a potential therapeutic agent for cardioprotection 20. Various solvents such as hexane, chloroform, methanol, ethanol and water were reported to be used to extract of the bark, seeds, flowers and leaves of Delonix regia plant and have been demonstrated to possess significant antiplasmodial activity in vivo 21. Moreover, methanol and acetone extracts of the flower have been also reported owned good larvicidal activities 22, 23. Meanwhile, Carica papaya L. extracted from leaf was reported having anti-proliverative against prostate cancer, antifungal activity, antibacteria, antidengue, anti-inflamatory and antimalaria 24-30 Here, we report our research which was designed to evaluate the bioactivity compounds from a combination of Delonix regia and Carica papaya L. leaves in ethanolic extract against malaria in Plasmodium berghei-infected mice as in vivo animal model which might be useful as potential remedies for malarial disease.
Method
Plant Preparation and Extraction
The plant material used in this study was obtained from Balitro (Tropical Plant Research), the leaves of the plant were cleaned, and shielded from sunlight, to obtain the dry ingredients, then mashed with a blender to get powder form. Extraction was performed by maceration for three days then supernatant was separated, it was added with 70% ethanol until all powder was dissolved. After 3 days immersion with stirring periodically, the solution is then filtered and pulp obtained by soaking several times until the filtrate was clear. The filtrate was concentrated using rotary evaporator at 50OC with 50 rpm and dried in the oven at 35OC to obtain a constant weight. Extracts were given to mice as treatments in three doses: 4.80 mg/g 9.75mg/g and 15.5 mg/g, respectively.
Phytochemistry Assays
Phytochemistry assays were conducted to determine secondary metabolites contained in the constrated extract of ethanol fraction of Delonix regia and Carica papaya L. Phytochemistry assays were carried out with the following procedure of Clule (1982) 31.The phytochemical analyses including glycoside, saponin, flavonoids, alkaloids, triterpenoid, steroids, essential oil and tannin assays.
Glycoside Assay
Glycoside phytochemistry assay was carried out by evaporating of 0.1 mL of sample solution over a water bath, then dissolve it with 5 mL acetic acid anhydride. Add 10 drops of concentrated sulfuric acid, the blue or green product is formed indicating glycosides.
Saponin Assay
Saponin screening is carried out by placing of 10 mL of sample solution in a test tube; then it is shaken vertically for 10 seconds and then left it to stand for 10 seconds, 1-10 cm tallfoam formation that is stable for less than 10 minutes indicated the presence of saponin. For the confirmation after the addition of 1 drop of 2N HCl, the foam does not disappear.
Flavonoids Assay
Flavonoids screening is done by placed of 1 mL of test solution was evaporated to dryness, the remaining moistened with acetone, and then added a fine powder of boric acidand oxalic acid, carefullyheated over a water bath and avoid overheating. Residual obtained is mixed with 10 ml.of ether. Observed with the ultraviolet 366 nm was yellow fluorescence in solution showed flavonoids.
Alkaloids Assay
About 2 ml. of sample solution was placed on a porcelain cup was evaporated to give of residue. The residue was added 5 mL of 2N HCl. The resulting solution is divided into 3 tubes. The first tube serves as a blank, was added HCl 2N, the second was added 3 drops of Dragendorff reagent, and the third tube was added 3 drops of Mayer reagent. Positive result of alkoloids characterized by the formation of an orange precipitate in the second tube and the yellow precipitate in the third tube.
Triterpenoid and Steroids Assay
The triterpenoid and Steroids assays were conducted using Liebermann Burchard reaction, 2 mL of test solution was evaporated in a pocelain cup. The residue was dissolved in 0.5 mL of chloroform and acetic acid anhydride was added. Then added 2 of concentrated sulfuric acid through the tube wall. The precence of triterpenoids is characterized by the formation of the brownish or violet ring at the boundary of the solution, where the presence of the steroids is characterized by the formation of blue-green ring.
Essential Oils Assay
About 1 mL of sample solution was pipetted and then evaporated on porclain disk to obtain a residue. Possitive result of the essential oil are characterized by a distinctive odor that is generated by the residue.
Tannin Assay
Tannin phytochemistry assay was conducted by reacting of 1 mL of the test solution with a solution of 10% of Iron(III) Chloride, the possitive result for tannin was shown by the formation of dark blue or greenish.
Inoculation of Plasmodium Berghei in Mice
The animals used in this experiment were 2-3 months old healthy male standard Swiss-Webster mice, weighing 20-30 grams. The mice were obtained from Centre for Research and Development of the Ministry of Health of the Republic of Indonesia. The mice used for antimalarial assay was initially infected with a donor of Plasmodium berghei. Two milliliters of infected blood from donor mice was taken from the heart by using a syringe which already contained anticoagulant. Moreover, the blood was collected in test tubes containing 3.8% of sodium citrate. Blood donors were collected and injected intraperitoneally in mice as much as 0.2 ml, then incubated for five days. After incubation, blood samples were taken from the mice tail vein in order to determine whether the mice had been infected.
Parasites were calculated at 500 erythrocytes and then the results were expressed in parasite density. The formula used in the parasite density calculation based on the formula given by Center for Disease Control and Prevention (CDC):
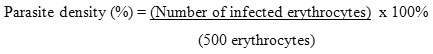
After obtaining the parasite density in each group, the increased parasitaemia could be calculated as below:
Increased parasitaemia (%) = Density H4 parasite – Parasite density H0
Percentage of inhibition of parasite density was determine based on the percentage of parasitaemia on the fourth day, followed Peter’s 4-day suppressive test. The standard calculation is recommended by the WHO to assess the potential antiplasmodium agent.
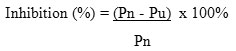
Descriptions
Pn = density of parasites average on day 4 in the negative control group
Pu = density of parasites average on day 4 in each test group
In Vivo Antiplasmodium Assay
Antiplasmodium activity test was performed according to the standard method of Peter’s Test (4-Days suppressive test). The blood preparations were prepared by placing one drop of blood sample, collected from the tail vein of mice into a glass microscope object. Another object glass was mounted on the preparation and the blood was smeared into a thin layer, with an angle of 30o. The layer air-dried, and then fixed in methanol absolute, followed by second drying. A few drops of in Giemsa dye was added to cover the entire surface. The preparation was allowed to stand for 30 minutes. Then preparation was washed with water, and the preparation was examined under a light microscope with a magnification of 10 x 100 multiplication. Mice that had been infected with the parasite were divided into 11 groups as shown in Table 1.
Table 1: In vivo treatments of Plasmodium berghei-infected mice with plant extracts.
| Mice group category | Part of plant extract |
(Dose mg sample/g mice body weight) |
| A1 | Delonix regia bark | 4.80 mg/20 g |
| A2 | Delonix regia bark | 9.75 mg/20 g |
| A3 | Delonix regia bark | 15.50 mg/20 g |
| B1 | Carica papaya L leaf | 4.80 mg/20 g |
| B2 | Carica papaya L leaf | 9.75 mg/20 g |
| B3 | Carica papaya L leaf | 15.50 mg/20 g |
| C1 | Delonix regia bark + Carica papaya L leaf | 1:1 |
| C2 | Delonix regia bark + Carica papaya L leaf | 1:3 |
| C3 | Delonix regia bark + Carica papaya L leaf | 3:1 |
| D
(water as negative control) |
– | – |
| E
(Sulfadoxine as positive.control) |
– | 1,36 mg/20 g |
Sample size used in this research was following Federer’s formula 31:
(k-1) (n-1) > 15
(11 – 1) (n – 1) > 15
n ~ 3
Descriptions
k = number of groups
n= number of mice in groups
Results
The phytochemical analyses of the ethanolic extracts of Delonix regia and Carica papaya L were done, qualitatively. The plants were collected and were identified. Then they were shade dried and powdered and were subjected to phytochemical screening. The qualitative chemical tests for the ethanolic extracts were performed. The investigation showed that Delonix regia contains flavanoids, alkaloids, triterpenoid and tannins. Meanwhile, Carica papaya L. screening showed that the ethanolic extract possesses glycosides, flavanoids, alkaloids, triterpenoid, steroids and tannins, as show on Table 1.
Table 2: Phytochemistry analysis extract ethanol of Delonix regia and Carica papaya L.
| Phytochemistry name | Delonix regia | Carica papaya L. |
| Glycosides | – | + |
| Saponin | – | – |
| Flavonoid | + | + |
| Alkaloid | + | + |
| Triterpenoid | + | + |
| Steroid | – | + |
| Essential oil | – | – |
| Tannin | + | + |
The treatment effect of sulfadoxine inhibition, ethanolic Delonix regia extract in Plasmodium berghei-infected mice, are tabulated in Table 1. Sulfadoxine induction in ill mice, showed 100% inhibition rates. Meanwhile, 9.75 mg/g Delonix regia extract showed 66.25% inhibition rate, followed by 4.80 mg/g Delonix regia and 15.50 mg/g Delonix regia extracts were 38.88% and 27.36%, respectively as depicted on Figure 1. In addition, Plasmodium berghei growth in mice mostly decreased by treatment of Delonix regia with concentration 9.75 mg/g as shown on Table 2.
Table 3: The growth and inhibition rates of parasite density on the 4th day of treatment with Delonix regia bark extract
| Treatment Group | % Growth | % Inhibition |
| Positive control | 0.00 | 100.00 |
| Delonix regia (4.80 mg/g) | 79.34 | 38.88 |
| Delonix regia (9.75 mg/g) | 42.35 | 66.25 |
| Delonix regia (15.50 mg/g) | 80.04 | 27.36 |
| Negative control | 100.00 | 0.00 |
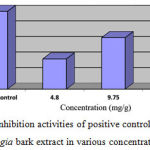 |
Figure 1: Inhibition activities of positive control and Delonix regia bark extract in various concentrations.
|
Table 4: The growth and inhibition rates of parasite density on the 4th day of treatment with Carica papaya L extract
| Treatment Group | % Growth | % Inhibition |
| Positive control | 0.00 | 100.00 |
| Carica papaya L (4.80 mg/g) | 19.43 | 83.75 |
| Carica papaya L (9.75 mg/g) | 29.00 | 89.02 |
| Carica papaya L (15.50 mg/g) | 27.02 | 72.89 |
| Negative control | 100.00 | 0.00 |
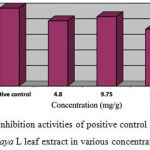 |
Figure 2: Inhibition activities of positive control and Carica papaya L leaf extract in various concentrations.
|
Treatment effect result of sulfadoxine inhibition in Figure 2 showed that ethanolic Carica papaya L extract in Plasmodium berghei-infected mice was found 100% inhibition rate. Meanwhile, 9.75 mg/g Carica papaya L extract showed 89.02%, followed by 4.80 mg/g Carica papaya L and 15.50 mg/g Carica papaya L extracts were 83.75% and 72.89%, respectively as depicted on Figure 1. In addition, Plasmodium berghei growth in mice mostly decreased by treatment of Carica papaya L with concentration 9.75 mg/g as shown on Table 3.
Table 5: The inhibition rates of parasite density on the 4th day of combination treatment with Delonix regia and Carica papaya L extracts
| Treatment Group | % Inhibition |
| Positive control | 100.00 |
| Combination 1:1 | 97.00 |
| Combination 3:1 | 29.03 |
| Combination 1:3 | 6.01 |
| Negative control | 0.00 |
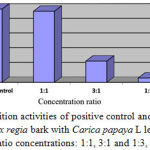 |
Figure 3: Inhibition activities of positive control and a combination of Delonix regia bark with Carica papaya L leaf extracts in various ratio concentrations: 1:1, 3:1 and 1:3, respectively.
|
Combination treatment of ethanolic Delonix regia and Carica papaya L extracts was also investigated its inhibition rates of parasite density on the 4th day as showed on Table 4. It showed that combination extracts in ratio 1:1 was found 97% the highest inhibition Plasmodium berghei in mice. Followed by Delonix regia bark with Carica papaya L leaf extracts in ratio concentrations 3:1 and 1:3 were 29.03% and 6.01%, respectively. It indicated that combination extracts in ratio 1:1 was potential bioactivities as well as sulfadoxine inhibition as positive control (Fig.3).
Discussion
The study of the bioactive compounds of the medicinal plants have acquired a lot of importance all over the world. The present study includes the phytochemical screening of the plants Delonix regia and Carica papaya L. The qualitative analyses of the ethanolic extracts from Delonix regia bark and Carica papaya L leaf showed the presence of phytochemical constituents, including flavonoid, alkaloid, triterpenoid, steroid, tannin and glycosides. These compounds are potentially used for various treatments as herbal remedies. Thus, the development of medicinal plants will be very useful for combating diseases 26, 33, 34.
The results of the study confirms the effectiveness of the use of extract plants is the easiest, reliable and the most practicable method, in inhibiting parasite density in Plasmodium berghei-infected mice. In this study, ethanolic Delonix regia bark extract potentially reduced parasite density down to 42.35% (0% growth rate with positive control), while in the previous study reported that bark and fruit peel extract of Delonix regia (72.8 mg/kg ) was found to be highly effective producing inhibition of 122% and 117% with hexane, chloroform, methanol, ethanol and water solvent extractions 33.
Meanwhile, ethanolic Carica papaya L leaf extracts potentially reduced parasite density down to 29% (0% growth rate with positive control). It is showed that Carica papaya L leaf extracts has higher inhibition activity compare to Delonix regia bark extract on the same single dose. The previous study reported that Carica papaya fruit and leaf extracts were potential antilarvicidal and antimalaria in vitro and in vivo 26, 33, 34.
Based on extract plants examination, it can be seen that the positive control has the highest inhibition rate. In clinical doses, the combination of Delonix regia and Carica papaya L. At 1:1 ratio has the highest antiplasmodium activity compared to other concentration ratios observed in this research. From previous studies it has been demonstrated that the bark of Delonix regia have antimalarial effects in mice infected with Plasmodium berghei. Phytochemical studies showed that Delonix bark extract contained natural compounds such as alkaloids, flavonoids, terpenoids, and phenolics 33.
A combination of two or more drugs or plant extracts can produce a synergistic or antagonist effects to Plasmodium berghei in mice. The synergistic effect occurs when the combination mutually enhance the efficacy of each ingredient. Antagonistic effect occurs when an ingredient in a combination reduces the activity of another ingredient, resulting in lower efficacy then in individual dose. If drugs or plant extracts combined do not interact, but only lead to accumulated effect, it is said that the combination has an additive effect. Thus, combinations of ethanolic extracts of Delonix regia and Carica papaya L in ratio 1:1 was giving the optimum anti-plasmodial activity (synergistic or additive effects) in vivo, which were suggested to be the effective medicinal plants antimalarial potential in mice.
Conclusion
In conclusion, thia Study has demonstrated potential anti-plasmodial activity for the treatment of malaria. The enhanced efficacy of certain combinations of herbal extracts remotely explains the practice and use of combined medicinal plant extracts in the management of malaria by traditional health users. This study revealed that combination of Delonix regia and Carica papaya L had much better anti-plasmodial properties compared to each single dose plant extract, and it should be subjected to further chemical and biochemical investigations. Moreover, natural products from plants used in traditional medicine, which have potent anti-plasmodial activity represent potential sources of new anti-malarial remedies. It is conceived further studies on these plants may lead to development of new affordable and effective phytomedicines for malaria.
Acknowledgement
We would like to thank Mr. Winarno and staff in Center for Research and Development of the Ministry of Health of the Republic of Indonesia for financially supported this study.
Conflict of Interest
Authors declare that there is no conflict of interest.
References
- Ramana, B. Malaria: who is at fault? World journal of surgery 31: 2072-2074 (2007).
CrossRef - Maguire, G.P. et al. Lung injury in uncomplicated and severe falciparum malaria: a longitudinal study in papua, Indonesia. The Journal of infectious diseases 192: 1966-1974 (2005).
CrossRef - Dale, P. et al. Malaria in Indonesia: a summary of recent research into its environmental relationships. The Southeast Asian journal of tropical medicine and public health 36: 1-13 (2005).
- Moorthy, V., Reed, Z. & Smith, P.G. Measurement of malaria vaccine efficacy in phase III trials: report of a WHO consultation. Vaccine 25: 5115-5123 (2007).
CrossRef - Syafruddin, D. et al. Malaria prevalence in Nias District, North Sumatra Province, Indonesia. Malaria journal 6: 116 (2007).
CrossRef - Yoda, T. et al. Evaluation by villagers of the malaria control project on Lombok and Sumbawa Islands, west Nusa Tenggara Province, Indonesia. The Southeast Asian journal of tropical medicine and public health 38: 213-222 (2007).
- Syafruddin, D. et al. Malaria in Wanokaka and Loli sub-districts, West Sumba District, East Nusa Tenggara Province, Indonesia. The American journal of tropical medicine and hygiene 74: 733-737 (2006).
CrossRef - Kondaparla, S. et al. Antimalarial activity of novel 4-aminoquinolines active against drug resistant strains. Bioorganic chemistry 70: 74-85 (2017).
CrossRef - Rajapakse, C.S. et al. Synthesis of New 4-Aminoquinolines and Evaluation of Their In Vitro Activity against Chloroquine-Sensitive and Chloroquine-Resistant Plasmodium falciparum. PloS one 10: e0140878 (2015).
CrossRef - Imwong, M. et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. The Lancet. Infectious diseases 17: 491-497 (2017).
CrossRef - Hanboonkunupakarn, B. & White, N.J. The threat of artemisinin resistant malaria in Southeast Asia. Travel medicine and infectious disease 14: 548-550 (2016).
CrossRef - Imwong, M. et al. An outbreak of artemisinin resistant falciparum malaria in Eastern Thailand. Scientific reports 5: 17412 (2015).
CrossRef - Fairhurst, R.M. Understanding artemisinin-resistant malaria: what a difference a year makes. Current opinion in infectious diseases 28: 417-425 (2015).
CrossRef - Nondo, R.S. et al. Anti-plasmodial activity of Norcaesalpin D and extracts of four medicinal plants used traditionally for treatment of malaria. BMC complementary and alternative medicine 17: 167 (2017).
CrossRef - Gathirwa, J.W. et al. The in vitro anti-plasmodial and in vivo anti-malarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. Journal of ethnopharmacology 115: 223-231 (2008).
CrossRef - Shabir, G. et al. Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf]. Molecules 16: 7302-7319 (2011).
CrossRef - Cheirmadurai, K., Thanikaivelan, P. & Murali, R. Highly biocompatible collagen-Delonix regia seed polysaccharide hybrid scaffolds for antimicrobial wound dressing. Carbohydrate polymers 137: 584-593 (2016).
CrossRef - Rahman, M. et al. Effect of Delonix regia leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. African journal of traditional, complementary, and alternative medicines : AJTCAM 8: 34-36 (2011).
- Shewale, V.D., Deshmukh, T.A., Patil, L.S. & Patil, V.R. Anti-Inflammatory Activity of Delonix regia (Boj. Ex. Hook). Advances in pharmacological sciences 2012, 789713 (2012).
CrossRef - Wang, L.S., Lee, C.T., Su, W.L., Huang, S.C. & Wang, S.C. Delonix regia Leaf Extract (DRLE): A Potential Therapeutic Agent for Cardioprotection. PloS one 11: e0167768 (2016).
CrossRef - Fatmawaty, F., Hendry Astuti Antimalarial activity of Delonix regia on mice with Plasmodium berghei. J Nat Prod. 6: 61–66 (2013).
- Deepa B, R.O.K. Larvicidal activity of the flowers of Delonix regia (Bojer ex Hook.) Rafin. (Fabales: Fabaceae) against the teak defoliator, Hyblaea puera. Cramer Curr Biotica 5: 237-240 (2011).
- Murthy J.M, R.P.U. Biological activity of certain botanical extracts as larvicides against the yellow fever mosquito, Aedes aegypti. L. J Biopestic 2: 72-76 (2009).
- Pandey, S. et al. Selective anti-proliferative activities of Carica papaya leaf juice extracts against prostate cancer. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 89: 515-523 (2017).
- He, X. et al. Chemical composition and antifungal activity of Carica papaya Linn. seed essential oil against Candida spp. Letters in applied microbiology 64(1): 350-354 (2017).
CrossRef - Okpe, O. et al. Antimalarial Potential of Carica papaya and Vernonia amygdalina in Mice Infected with Plasmodium berghei. Journal of tropical medicine 2016, 8738972 (2016).
CrossRef - Charan, J., Saxena, D., Goyal, J.P. & Yasobant, S. Efficacy and safety of Carica papaya leaf extract in the dengue: A systematic review and meta-analysis. International journal of applied & basic medical research 6: 249-254 (2016).
CrossRef - Pandey, S., Cabot, P.J., Shaw, P.N. & Hewavitharana, A.K. Anti-inflammatory and immunomodulatory properties of Carica papaya. Journal of immunotoxicology 13: 590-602 (2016).
CrossRef - Ansari, R.M. Extract of Carica papaya L. leaves: Standardising its use in dengue fever. Indian journal of pharmacology 48: 338-339 (2016).
CrossRef - Mbosso Teinkela, J.E. et al. In vitro antimicrobial and anti-proliferative activities of plant extracts from Spathodea campanulata, Ficus bubu, and Carica papaya. Pharmaceutical biology 54: 1086-1095 (2016).
CrossRef - Ciulei, I. Methodology for analysis of vegetable drugs, practical manual on the industrial utilisation of medicinal and aromatic plants. Phytochemistry 63: 62(1) 97-104 (1982).
- Federer, W. Statistics and Society: Data Collection and Interpretation. 2th Edn., CRC Press, New York, Pages: 600 (1991).
- Fatmawaty, F., Astuti, H. Antimalarial activity of Delonix regia on mice with Plasmodium berghei. Journal of natural products 6: 61-66 (2013).
- Thomas, T.G., Raghavendra, K., Lal, S. & Saxena, V.K. Mosquito larvicidal properties of latex from unripe fruits of Carica papaya linn. (Caricaceae). The Journal of communicable diseases 36: 290-292 (2004).







