P. Udaya Sri¹, K. M. Subbu Rathinam² and K. R. S.Sambasiva Rao¹
¹Department of Biotechnology, Acharya Nagarjuna University, Nagarjuna Nagar - 522 510 India.
²Department of Zoology, Rajah Serfoji Govt.College, Thanjavur - 613 005 India.
Abstract
Cotton is the world’s most important non-food agricultural commodity, which is easily damaged by various sucking pests like jassids, aphids, thrips, whitefly, red spider, mites, mealy bug and bollworms. Pesticides and their residues pose a major problem by their accumulation in different parts of the world in general and in different biological organisms of the food chain in particular. In the light of the importance and essentiality to overcome the deleterious effects of pesticides, the present study has made an attempt to isolate the fungal species capable of degrading organophosphorus pesticides (chlorpyriphos, malathion and triazophos) that are widely sprayed on cotton crop in Andhra Pradesh and to develop a model for the control of pesticide pollution. The isolated species were identified basing on microscopic structural and growth characterization studies together with scanning electron microscopy and the molecular characterization was also done basing on the sequence analysis of the DNA coding for 18s rRNA of the test fungal organism. The results may fit the findings of others on the ability of some fungi to degrade pesticides and introduce some new information on mineralization of the organic constituents of pesticides. It was elucidated that the selected fungal species is an effective biodegrading agent and by optimizing the different cultural conditions and by using large scale culture techniques, this fungal species, Aspergillus niger, can be very effectively used as biodegrading agent in cotton fields as effective biocleaner and will be an economically effective microbial process which is an alternative for control of environmental pollution.
Keywords
Pesticides; Organophosphates; Biodegradation; Environmental pollution
Download this article as:| Copy the following to cite this article: Sri P. U, Rathinam K. M. S, Rao K. R. S. S. Detoxification of Chlorpyriphos Using Fungal Species Aspergillus Niger from Cotton Soils of Andhra Pradesh, India. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Sri P. U, Rathinam K. M. S, Rao K. R. S. S. Detoxification of Chlorpyriphos Using Fungal Species Aspergillus Niger from Cotton Soils of Andhra Pradesh, India. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1559 |
Introduction
India is primarily an agriculture-based country with more than 60-70% of its population dependent on agriculture. A tremendous pressure will be exerted on the annual food grain production and on minimizing the crop losses. About 30% of agriculture produce is lost due to pests. Hence agrochemicals like insecticides, fungicides, pesticides and herbicides have increasing demand for protection of crops in one way or the other and large numbers of pesticides are emerging out day by day.
Cotton is the world’s most important non-food agricultural commodity, but in India it is one of the important commercial fiber crops of South India. It is the back bone of textile industry accounting for 70% of total fiber consumption in the textile sector and 38.5% of the country’s export, fetching income of Rs. 42,000 crores. Cotton is easily damaged by various sucking pests like jassids, aphids, thrips, whitefly, redspider, mites, mealy bug and Bollworms (Pimentel, 1983). This accounts for the consumption of more amounts of insecticides on cotton than any other single crop that is the reason why cotton growers are always looking for ways to protect their crops. There is a tremendous need to increase the agricultural production to meet the requirement of increasing population. Pesticides have gained an immense importance for the very effective control of agricultural pests which spoils 1/3rd of food production. The indiscriminate use of pesticides for pest control has resulted in unprecedented chemical pollution and simultaneously affecting the non-target organisms (Kurzel and Cetrulo, 1981; Hayes, 1986). Pesticides and their residues pose a major problem by their accumulation in different parts of the globe in general and in different biological organisms of the food chain in particular (Singh et al., 1987).
In the light of the importance and essentiality to overcome the deleterious effects of pesticides, an attempt has been made in the present study to investigate the possibility of biodegradation of Chlorpyriphos using fungal species and to develop a model for control of pesticide pollution.
Material and Methods
Distribution of cotton cultivable area in Guntur District of Andhra Pradesh
A Survey of cotton cultivable area was carried out in Guntur District of Andhra Pradesh. Soil samples were collected from different areas by adopting simple random sampling technique to isolate the pesticide degrading fungi from the soils where tremendous pesticide consumption had taken place. Soil samples were collected from Siripuram, Prathipadu, Chilakaluripet and Amaravathi of Guntur District, which is the leading district in the cultivation of cotton in Andhra Pradesh. Information was obtained from farmers regarding the extensively sprayed pesticides on cotton crop.
Isolation and identification of fungal strains
The soil samples were collected at a depth of 15 inches depth from the soil where there was high moisture content, because the fungi need moisture content for growth. The soil sample collected was dispensed in the sterile bags and sealed and the samples were brought to the laboratory and were added with 1% of the selected insecticide in addition to controls. After incubation for 4 weeks at 28ºC, soil samples for isolation of fungi were taken using the dilution plate method. The isolated fungi were identified using Lactophenol cotton blue staining technique, scanning electron microscopy and molecular techniques.
Lactophenol cotton blue Staining
The fresh culture of different age starting from 5 to 25 days was used for the preparation of semi-permanent slides. A small bit of mycelium was placed on the slide and mixed with a drop of Lacto phenol cotton blue. Then it was slightly heated on the steam and fixed. Finally the stained slide was covered with cover slip and observed under binocular compound microscope.
Scanning electron microscopy of test fungi
Scanning Electron microscopic studies was carried out by taking 24 h old cultures of test fungi and fixed in 6% buffered Glutaraldehyde followed by post fixation in Osmium tetroxide and then dehydrated in increasing concentration of Ethyl alcohol. The samples were mounted on copper stubs with double sided adhesive tape, coated with gold polaron, AU/PD sputter-coater and scanned in SEM (Jeol JSM 5600, Japan) and photographed. The SEM studies were conducted at Ruska laboratories, Sri Venkateswara Veterinary University, Rajendranagar, Hyderabad.
Identification of the isolated fungi by sequencing of the amplified 18S rRNA gene
The most powerful tool to identify the unknown fungal species is to sequence the gene (DNA) coding for 18S rRNA, which is present in the genome of the fungi. The gene coding for the 18S rRNA is amplified using the Polymerase Chain Reaction (Mullis, 1990), and the amplified product has been subjected to sequencing and the sequence obtained has been compared with the sequence obtained from the Nucleotide Database of NCBI.
Studies on the Growth of Selected Test Fungi
Determination of Growth
Growth pattern of the strain was studied in Potato Dextrose broth, Czapekdox broth & Sabourauds broth. The isolated fungal strain was inoculated into the broth and the culture was incubated at 200C. At every 24hrs interval, growth was noticed. The growth of the culture in broth is noticed by taking the spore counts and dry weights at successive time intervals using the following formula:
Study of the Effect of Physico-Chemical Parameters (Temperature, PH and Salt Concentration) On the Growth of Organophosphate Degrading Fungi
The isolated fungal strain was inoculated into the Potato Dextrose broth and the culture was incubated at 200C. To assess the growth at varied pH, the pH was adjusted as 4, 5,6,7,8 and 9. To assess the growth at varied salt concentrations, the salt concentrations were adjusted as 0%, 0.5%, 5%, 7.5% and 10%. Then the flasks were autoclaved at 15 lbs pressure for 20 minutes at 1210C. To assess the growth at varied temperatures, the temperatures were adjusted as 250C, 280C, 300C, 350C, 370C, 400 C, 450C, 550C and incubated for 4 days (Aneja, 2003).
Confirmation of Pesticide Biodegrading ability of Fungi Aspergillus niger
Chlorpyriphos, the most widely used Organophosphate pesticide in Guntur district, particular for cotton cultivation to control cotton leaf worm was selected for the degradation studies. The isolated fungal strain was inoculated into the Potato Dextrose broth and CzapekDox medium and the culture was incubated at 200C. At every 24hrs interval, growth was noticed. The growth of the culture in broth is noticed by taking the spore counts and dry weights at successive time intervals. The amount of the pesticide residue in the samples was analyzed through GC technique. Residual pesticide and pesticide breakdown products extracted from the microbial degradation studies were quantified by GLC methods, using Varian- model CP 3800 with a tritium source electron capture detector and a Tracor model MT-220 with a Melpar flame photometric detector (pulsated flame photometric detector) (Ramesh Babu et al., 2001).
Results and Discussion
Although it is well established that the microorganisms are capable of degrading pesticides and present in all types of environment(s), still environmental pollution is a persistent problem and many areas are highly contaminated with pesticides and other chemicals (Alexander, 1994). Their susceptibility to biodegradation change drastically, depending on several factors related to the chemical and physical properties of both the pesticides and the environment in which they are present. Based on these facts, our investigation was aimed at studying the biodegradation of Chlorpyriphos using the selected fungal species Aspergillus niger isolated from the cotton soils of Andhra Pradesh, India.
A survey was conducted during the year 2005-2006 in various cotton cultivable areas of Guntur District of Andhra Pradesh for determining the intensity of pesticide utilization through direct interaction with the cotton farmers (Table 1).
Table 1: Survey of Pesticides used in different places.
Different soil samples were collected from different locations of cotton cultivated areas of Guntur District. As the soil contain different useful bacterial and fungal species, in our present investigation, the soil samples from different areas were screened to identify the various bacterial and fungal species present in the samples (Table 2). These soil samples consist of various fungal species of the genus Aspergillus, Fusarium, Penicillium, Rhizopus, Trichoderma etc., The fungal isolates were cultured in the presence of the selected widely used organophosphorus pesticides.
Table 2: Microorganisms collected in various soil samples of Guntur district
| 1 | Aspergillus niger, | Bacillus subtilis, | Bacillus subtilis, | E.coli, Penicillium |
| Saccharomyces | Aspergillus flavus, | Aspergillus niger, | notatum, Bacillus | |
| cerevisiae, Fusarium | Saccharomyces | Trichoderma viride | subtilis | |
| oxysporum | cerevisiae | |||
| 2 | E.coli, Pseudomonas | Aspergillus terres | Bacillus subtilis, | Bacillus subtilis, |
| putida, Penicillium | Pseudomonas | Aspergillus niger | Saccharomyces | |
| chrysogenum | aeruginosa, Mucor | Mucor racemosus | cerevisiae, | |
| racemosus, Bacillus | Rhizopus | Fusarium solani | ||
| Subtilis | microsporus | , Mucor indicus | ||
| 3 | Bacillus cereus, | Penicillium | Penicillium | Pseudomonas |
| Fusarium solani, | chrysogenum, | chrysogenum, | aeruginosa, E.coli, | |
| Fusarium solani, | Fusarium | Fusarium | ||
| Aspergillus flavus | Bacillus subtilis, | oxysporum, Mucor | oxysporum | |
| Trichoderma viride | racemosus. | |||
| 4 | Mucor racemosus, | Rhizopus | E.Coli, Pseudomonas | Saccharomyces |
| Trichoderma viride, | microsporus, E.Coli, | putida, Fusarium solani | cerevisiae, Mucor | |
| E.Coli | Bacillus subtilis | Rhizopus microsporus | indicus, | |
| Fusarium oxysporum | Saccharomyces | Trichoderma viride | ||
| Pseudomonas putida, | cerevisiae | |||
| 5 | E.coli, Pseudomonas | Bacillus cereus | Pseudomonas putida, | Bacillus subtilis, |
| aeruginosa, | Pseudomonas | Bacillus subtilis, | Mucor indicus, | |
| Rhizopus microsporus | aeruginosa, | Fusarium | Aspergillus flavus, | |
| Aspergillus niger | Rhizopus | oxysporum, | E.Coli | |
| microsporus, | Aspergillus niger | |||
| Fusarium oxysporum | ||||
| 6 | Achromobacter, | Bacillus subtilis | Bacillus subtilis, | E.coli, |
| Bacillus subtilis, | E.Coli, | E.Coli, | Rhizopus | |
| Penicillium notatum | Aspergillus niger | Rhizopus, | microsporus,, | |
| Saccharomyce | Pseudomonas | microsporus, | Saccharomyces | |
| s cerevisiae | aeruginosa | Penicillium, | cerevisiae | |
| chrysogenum | ||||
| 7 | Mioxococcus, | Mucor racemosus, | Bacillus subtilis, | E.coli, |
| Alternaria solani, | Aspergillus flavus, | Micrococcus, | Pseudomonas | |
| Aspergillus niger, | Penicillium | Fusarium solani, | putida, Penicillium | |
| Trichoderma viride | chrysogenum | Mucor racemosus , | notatum, | |
| Trichoderma | ||||
| viride | ||||
| 8 | Bacillus subtilis, | E.Coli, | Pseudomonas putida, | Bacillus subtilis, |
| Pseudomonas putida, | Rhizopus | Bacillus subtilis, | E.coli, | |
| Saccharomyces | ||||
| Rhizopus stolanifer | microsporus, | Mucor racemosus | cerevisiae, | |
| Trichoderma viride | Fusarium solani | Aspergillus terres | Fusarium solani | |
| 9 | E.coli, | Bacillus subtilis | Achromobacter, | Pseudomonas |
| Aspergillus flavus | Pseudomonas | Penicillium notatum | putida, | |
| Fusarium solani | aeruginosa, | Rhizopus microsporus | Alternaria solani, | |
| Aspergillus flavus | Saccharomyces | Fusarium | ||
| cerevisiae | oxysporum | |||
| 10 | Pseudomonas | Bacillus subtilis, | Pseudomonas putida, | Bacillus cereus |
| aeruginosa, | Myxococcus, | Aspergillus niger, | E.coli, | |
| Aspergillus terres | Penicillium notatum | E.Coli | Aspergillus flavus | |
| Mucor indicus | ||||
Microscopic structural and growth characterization, scanning electron microscopic studies and molecular characterization basing on DNA coding for 18s rRNA sequences of the test organisms capable to degrade pesticides were performed and it was concluded that the test fungus is Aspergillus niger. It produces black colonies. Aspergillus niger (Fig.1) was categorized into three groups namely fluffy and medium, elevated and abundant and pulverulent and scanty The optimal conditions for germination were 25oC and pH 5.0 on Potato Dextrose Agar (PDA) the colonies are wooly and become compact in time (Samson et al., 2001). The isolate Aspergillus was identified to the species level based on the mycelial and cultural characters with the help of standard descriptions given by Alexopoulos and Mims (1979). Basing on the microscopic observation and culture characteristics, it can be concluded that the screened and identified species were confirmed to be Aspergillus niger.
 |
Figure 1: Micrograph of Aspergillus niger |
The studies on Aspergillus niger using Scanning electron microscopy revealed that the isolated species contain mycelial characters of Aspergillus niger (Fig.2). The hyphae are well developed, profusely branched, septate and hyaline. The cells are multinucleate. The conidiophores are long and erect. Conidium bearing sterigmata are bottle shaped. The conidia are globose and unicellular.
 |
Figure 2: Scanning Electron Micrograph of Aspergillus niger.
|
The scanning electron microscopic studies confirmed that the screened species were confirmed to be as Aspergillus niger. As the microscopic structural identification and the growth characterization cannot be attributed to confirmed degradative capacity of the fungal organisms, it requires further characterization. Hence, the fungal confirmation study was extended using molecular techniques based on the DNA coding for 18s rRNA sequences.
The genomic DNA of the isolated fungal organisms was subjected for the isolation of DNA coding for 18s rRNA by using PCR (Fig.3). The bands were cut and eluted and the DNA so obtained was subjected for sequencing.
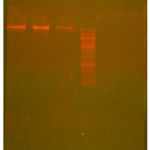 |
Figure 3: Agarose gel showing Genomic DNA.
|
The sequencing analysis of isolated DNA has revealed that the amplified DNA corresponds to 600 base pairs for Aspergillus niger. The DNA sequence obtained for the different isolated fungi is shown in Fig. 5.
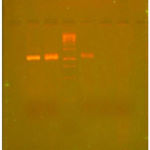 |
Figure 4: Agarose gel showing the amplified 18sDNA.
|
The sequences obtained in the present investigation were subjected to BLAST hit with the sequences of NCBI database. Basing on the alignment occurred, aligned sequences at the maximum degree were selected and the phylogenetic tree was constructed. The phylogenetic tree so obtained was given below (Figure 6).
 |
Figure 5: 18S ribosomal RNA gene of Aspergillus niger.
|
The microscopic structural and growth characterization studies together with scanning electron microscopic studies and amply supported by the molecular characterization basing on the sequence analysis of DNA coding for 18s rRNA of the test fungal organisms, it was confirmed that the isolated fungi belongs to Aspergillus niger (Fig. 5) and further confirmed that these fungi are capable to degrade the organophosphorus pesticides. The study was extended to develop an effective biodegradation model.
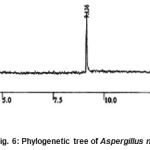 |
Figure 6: Phylogenetic tree of Aspergillus niger.
|
Growing fungi on laboratory media is a costly and tedious process. From the observations, it was evident that the Potato Dextrose broth has produced more number of spores followed by Czapekdox broth and least number of spores was produced in Sabourauds broth.
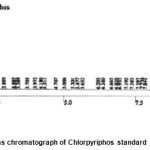 |
Figure 7: Gas chromatograph of Chlorpyriphos standard. |
The OP pesticides are known to cause adverse effects to the ecosystem and mankind. They affect the nervous system by inhibiting a critical enzyme called acetylcholinesterase, involved in nerve signaling. An appropriate balance of acetylcholinesterase is needed to help with muscle control, including muscles of the respiratory tract, heart, and arms and legs. The OP pesticides can cause damage to the reproductive system (sperm defects in men, and decreased pregnancies and survival of offspring in animals) chest pain, respiratory distress, incoordination, vomiting, damage to the central and peripheral nervous systems and death due to respiratory distress or cardiac arrest at high levels of exposure. Keeping the significance of effective biodegradation of OP pesticides by fungi, in the present study an attempt was made to optimize the various physico-chemical parameters for efficient growth of cultured fungal organisms and inturn to enhance the biodegradation.
 |
Figure 8: Gas chromatograph of Chlorpyriphos control.
|
 |
Figure 9: Gas chromatograph of Chlorpyriphos sample
|
After inoculation of Aspergillus niger in the Potato Dextrose broth, incubated at different temperatures (25°C, 28°C, 30°C, 35°C, 37°C, 40°C, 45°C and 55°C) for 4 days, maximum growth was observed at temperatures of 250C, 280C and 300C whereas minimal growth was observed at temperatures 35°C and 37°C and there was no growth above temperatures of 400C. This reveals that the optimum temperature for Aspergillus niger was 25°C – 30°C (Table 4).
Potato Dextrose broth with different pH (2.5, 3.5, 4.5, 5.5, 6.5, 7, 7.5, 8.5, 9.5) was inoculated with Aspergillus niger and incubated for 7 days. Growth was not observed in the flasks with pH 2.5, and 3.5. Maximum growth was observed at pH 5.5 and moderate growth was observed at pH 4.5, 6.5, 7.0, 7.5 and 8.5 and no growth was observed at pH 9.5. These results indicate that the optimum pH for the growth of the selected fungus at these conditions was 5.5 and moderate growth in between 6.5 to 8.5 (Table 4).
Growth medium with different salt concentrations (0%, 0.5%, 5%, 7.5% and 10%) were inoculated with Aspergillus niger and incubated for 7 days. The growth was observed in all the inoculants of NaCl concentration of 0%, 0.5% and 2.5% where as the growth was not observed in 5%, 7.5% and 10% of NaCl concentration. This gives the information that the growth possibility for our desired fungi was upto 2.5% Sodium chloride (NaCl) concentration only (Table 4).
The degradation capability of selected fungal species, Aspergillus niger on selected organophosphate pesticide, Chlorpyriphos was confirmed through Gas chromatography. The biodegradation of Chlorpyriphos with the above selected fungal species for medium composition for each combination of a particular species and pesticide was investigated.
Chlorpyriphos was degraded to the maximum extent by Aspergillus niger. In both the media, i.e., Potato Dextrose broth and Czapekdox medium, similar pattern of degradation of the respective organophosphate pesticides was noticed. It was also evident that the maximum percentage of degradation was seen in Potato Dextrose broth by the test fungi on the used organophosphate pesticides (Table.5). The pesticide residues were extracted from the culture medium and the analysis of the pesticide residue samples was carried out by using Gas chromatography technique.
It was clearly evident that the cotton cultivated soils are prone for heavy pesticide residues due to the continuous usage and it is quite possible that these pesticides can enter into food chain and can cause several deleterious effects even to human beings. Results of the present study may fit the findings on the ability of fungi to degrade pesticides and introduce some new information on mineralization of organic constituent of pesticides. This will decrease the side effects of pesticides on the non-target microorganisms that play an important role in soil fertility. In the light of this, an attempt has to be made further, to investigate the possibility of biodegradation of various organophosphorus pesticides using the fungal isolate Aspergillus niger.
Conclusion
The study elucidated that the selected fungal species can be employed as effective biodegrading species. By further optimizing the different culture conditions, the selected fungal species, Aspergillus niger can be used as an effective biodegrading agent in cotton fields. Also it was found to be an efficient alternative for the control of environmental pollution as it is screened from the soils with 7-8 years of cotton growing history and was isolated in presence of the selected pesticide.
References
- Alexander M., Biodegradation and Bioremediation, Academic Press, San Diego, 139 (1994).
- Alexopoulos C. J. and Mims C.W., Introductory Mycology, 3rded. John Wiley and Sons, New York (1979).
- Aneja, K.R., Experiments in Microbiology, Plant Pathology, Tissue Culture and Mushroom Production Technology; New Age International Publishers; Third Edition. 221-240 (2003).
- Hayes, W.J., Pesticides Studied in Man. Baltimore, MD: Williams and Wilkins, Baltimore, 49 (1982).
- Kurzel, R.B., Certrulo, C.L., The effect of environmental pollutants on human reproduction, including birth defects. Environ. Sci. Technol. 15: 626-631 (1981).
- Mullis, K.B., The Unusual origin of Polymerase Chain reaction. Scientific American. April: 36-43 (1990).
- Pimentel, D., Effects of pesticides on the environment. 10th Intl. Congress on Plant Protection. Crydon, U.K. 2: 685-691
(1983). - Ramesh Babu, T., Srinivasa Rao, Ch., Narasimha Reddy, K., Temperature programmed gas chromatography method for the determination of pesticide residues. Indian Journal of Plant Protection. 29(1 & 2): 161-164 (2001).
- Samson R.A., Houbraken J., Summerbell R.C., Flannigan B. and Miller J.D. Common and important species of fungi and actinomycetes in indoor environments. In: Microogranisms in Home and Indoor Work Environments. New York: Taylor & Francis. 287-292 (2001).
- Singh, V.K., Singh, A., Singh, S.P., Effect of interaction of herbicides on chlorophyll and mineral content of rice leaves. J. Agric. Res. 2: 175–177 (1987).







