Omar M. E. Abdel-Salam1, Eman R. Youness2 and Walaa A. Abu-Elhamed3
1Department of Toxicology and Narcotics, National Research Centre, Cairo, Egypt.
2Department of Medical Biochemistry, National Research Centre, Cairo, Egypt.
3Department of Pediatrics, Faculty of Medicine, Cairo University, Cairo, Egypt.
Corresponding Author E-mail: omasalam@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1153
Abstract
Autism is a neurodevelopmental disorder of early childhood with unknown aetiology. In this study we aimed to investigate the changes in biochemical markers of inflammation, apoptosis, and mitochondrial function in the serum of children affected with autism spectrum disorder. Moreover we evaluated the changes in cholinesterase activity as a cholinergic marker in serum of these subjects. Twenty autistic children aged 3 to 12 years were gender and age-matched with 20 typically developing (TD) children. Changes in the levels of the proinflammatory cytokine monocyte chemoattractant protein-1 (MCP-1), transcription factor nuclear respiratory factor 2 (NRF-2), the antiapoptotic factor -cell leukemia/lymphoma 2 (Bcl2) as well as cholinesterase activity were measured in serum of autistic children and controls. We found significant increments in serum MCP-1, NRF-2 and Bcl2 of autistic children by 185.3%, 41.8% and 63.5%, respectively, compared to corresponding control values. There was also marked increase in serum cholinesterase activity by 97.5% (P<0.001) in autistic patients compared to controls. These results indicate an increased inflammatory response in serum of autistic children and suggest that serum levels of BChE, Bcl2 and NRF-2 are elevated in autism, possibly as an adaptive mechanism to the chronic inflammatory process. Serum BChE might serve as a biomarker of inflammation in autistic subjects.
Keywords
Autism; inflammation; mitochondrial dysfunction; serum cholinesterase
Download this article as:| Copy the following to cite this article: Abdel-Salam O. M. E, Youness E. R, Abu-Elhamed W. A. Changes in Monocyte Chemoattractive Protein, Nuclear Respiratory Factor 2, B-Cell Leukemia/Lymphoma 2 and Cholinesterase in Serum of Autistic Children. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Abdel-Salam O. M. E, Youness E. R, Abu-Elhamed W. A. Changes in Monocyte Chemoattractive Protein, Nuclear Respiratory Factor 2, B-Cell Leukemia/Lymphoma 2 and Cholinesterase in Serum of Autistic Children. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15146 |
Introduction
Autism is a neurodevelopmental disorder of early childhood characterized by behavioral abnormalities, impairments in communication, attention, cognition, learning, social interactions, and repetitive stereotypic behaviors.1,2 More boys than girls are affected with a ratio of 4:13 with a prevalence rate of 1 in 68 births in US4. The exact cause of autism is not yet fully understood, but genetic factors,5 immunological dysfunction,6 allergy7 and environmental agents e.g., diet, mercury, and infection with measles8,9 have all been suggested to contribute to its aetiology. In autistic children, there are increased autoantibodies against specific dietary peptides, bacterial antigens, mercury8,9 and also against brain proteins eg., anti-myelin-associated glycoprotein antibodies10 and antinucleosome antibodies.6 In response, oxidative stress,11-13 increased cytokine production and inflammation14-16 are detected in brain and serum of autistic subjects and are likely to mediate tissue damage.17 Cholinergic deficit underlying social impairment in autism has also been suggested.18 Moreover, deficits in mitochondrial bioenergetics19 and evidence of oxidative damage to the mitochondria12,20 are found in autism. Autistic children also show structural brain changes such as increased cerebral volumes21 and decreased cortical matter in specific brain regions.22
In this study, we measured the levels of the proinflammatory cytokine monocyte chemoattractant protein-1 (MCP-1), and the transcription factor nuclear respiratory factor 2 (NRF-2) in serum of autistic subjects. Nuclear respiratory factor 2 (NRF-2) is a transcription factor that activates mitochondrial genes involved in electron transport and oxidative phosphorylation23 and both NRF-1 and NRF-2 act to regulate mitochondrial energy metabolism critical for neuronal function.24 We also measured the changes in the antiapoptotic factor -cell leukemia/lymphoma 2 (Bcl2) in serum. The Bcl2 family of proteins is important in maintaining mitochondrial membrane integrity and in regulating the mitochondrial pathway of apoptosis. The antiapoptotic protein Bcl-2 acts by preventing the redistribution of the proapoptotic protein Bax to the mitochondria and thereby prevents the release of cytochrome c into the cytosol and the consequent activation of caspase proteins that initiate apoptosis.25 Moreover, the level of cholinergic marker butyrylcholinesterase (BChE) activity was measured in the serum of autistic individuals.
Patients and Methods
Patients Selection
This cross sectional case-control study included twenty autistic children and adolescents (15 males and 5 females; age range, 3-12 years) with a mean age 5.67 ± 0.59 years. Subjects were diagnosed according to the 4th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM IV).26 Diagnosis was done by a child psychiatrist. Subjects were recruited from Pediatric Psychiatry Clinic, Children’s hospital, Faculty of Medicine, Cairo University, during the period from 2013-2014. None of the patients had underlying conditions apart from autism eg., syndromic causes, chromosomal or metabolic abnormalities. Autistic subjects were compared to 20 healthy age- sex- and pubertal stage-matched children and adolescents serving as controls. The latter had no clinical findings suggesting neuropsychiatric manifestations, any organic health problems or medications affecting our result. An informed written consent of participation in the study was signed by the parents or legal guardians of the studied subjects. The study was approved by the Bioethical Research Committee, Faculty of Medicine, Cairo University hospitals, Egypt.
Laboratory Investigations
Quantification of MCP-1
Monocyte chemoattractant protein-1 was measured in serum using commercially available human MCP-1 ELISA kit (Glory Science Co., Ltd., Del Rio, TX, USA) according to manufacture instructions. The kit uses a double antibody sandwich enzyme linked immunosorbent assay to measure the level of MCP-1.
Quantification of NRF-2
Nuclear respiratory factor 2 was assayed in serum using a double-antibody sandwich enzyme-linked immunosorbent assay (Shanghai Sunred Biological Technology Co., Ltd, Jufengyuan Road, Baoshan District, Shanghai).
Quantification of Bcl2
B-cell leukemia/lymphoma-2(Bcl2) was measured in serum using ELISA Kit purchased from Glory Science Co., Ltd. (Del Rio, TX, USA).
Determination of BChE Activity
Butyrylcholinesterase (BChE) activity in serum was measured using a commercially available kit from Ben Biochemical Enterprise (Milan, Italy).
Statistical Analysis
Data are presented as mean ± SEM. Statistical analysis of the data was done using Student’t test with SPSS software (SAS Institute Inc., Cary, NC). A probability value of less than 0.05 was considered statistically significant.
Results
Serum MCP-1 concentrations were significantly higher by 185.3% (p<0.001) in autistic subjects (234.9 ± 8.9 ng/l) than in the control group (82.32 ± 6.0 ng/l) (Figure 1). Serum NRF-2 increased by 41.8% (p<0.001) from a mean of 32.15 ± 2.5 ng/ml in the control group to 45.6 ± 2.3 ng/ml in the autistic group (Figure 2). Serum Bcl2 was significantly higher in autistic subjects than in the control group (63.5% increase: 2.06 ± 0.18 ng/ml vs. 1.262 ± 0.13 ng/ml, p<0.001) (Figure 3). Serum butyrylcholinesterase (BChE) activity increased from a mean control value of 3872.9 ± 166.5 U/l to 7648.8 ± 171.4 U/l in those with autism (p<0.001) (Figure 4).
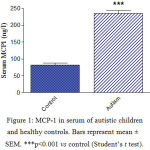 |
Figure 1: MCP-1 in serum of autistic children and healthy controls. Bars represent mean ± SEM. ***p<0.001 vs control (Student’s t test). |
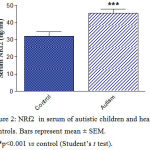 |
Figure 2: NRf2 in serum of autistic children and healthy controls. Bars represent mean ± SEM. ***p<0.001 vs control (Student’s t test). |
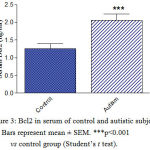 |
Figure 3: Bcl2 in serum of control and autistic subjects. Bars represent mean ± SEM. ***p<0.001 vs control group (Student’s t test). |
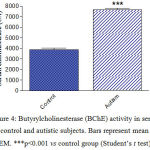 |
Figure 4: Butyrylcholinesterase (BChE) activity in serum of control and autistic subjects. Bars represent mean ± SEM. ***p<0.001 vs control group (Student’s t test). |
Discussion
The present study provided further evidence for an increased inflammatory response in children and adolescents with autism spectrum disorder. Thus a marked increase in serum MCP-1 level was observed in autistic subjects compared with their controls. This inflammatory chemokine is involved in the recruitment of monocytes and other phagocytic cells eg., macrophages and microglia into the sites of inflammation and tissue damage.27,28 MCP-1 increases in brain tissue of subjects with autism driven by the activation of monocytes and astrocytes and which indicates the presence of an active neuroinflammation in this disorder.29 Serum levels are also elevated in children with autism compared with typically developing counterparts and appear to correlate with executive functioning.30 Moreover, Ashwood et al.14 demonstrated an association between the increase in MCP-1 in autistic children and impaired behaviors and impaired developmental and adaptive functioning. MCP-1 could be induced under conditions of mildly impaired oxidative metabolism, causing the recruitment and activation of microglia to produce cytokines and resulting in neuronal death.31 The chemokine is fundamental to neuroinflammation since MCP-1 deficient mice exhibited decreased microglia activation and lower brain inteleukin-1 β and tumour necrosis factor-α in response to systemic lipopolysaccharide injection.32 MCP-1 levels are thus elevated in other neurological conditions characterized by tissue damage and/or inflammation eg., traumatic brain injury,28 ischaemic stroke33,34 and multiple sclerosis.35
Mitochondrial dysfunction has been implicated in the pathogenesis of autistic disorders.20,36 Mitochondria represent an important source for endogenous reactive oxygen metabolites and are also a target for free radicals-mediated oxidative damage.37 Mitochondrial abnormalities in autism included reduced glutathione reserve capacity, increased free radical generation and a greater decrease in mitochondrial membrane potential upon exposure to physiologic concentrations of nitric oxide.38 Napoli et al.20 reported decreased oxidative phosphorylation capacity of granulocytes from autistic children which might result from oxidative damage to the mitochondria by the excessive production of reactive oxygen metabolites. This latter view was supported by the presence of mitochondrial DNA damage and a lower gene expression of the nuclear factor erythroid 2-related factor 2 (Nrf2). The transcription factor Nrf2 regulates cellular resistance to oxidants via controlling the expression of antioxidant response element-dependent genes and thus protect the cells against oxidative stress.39,40 Other researchers indicated abnormal mitochondrial reserve capacity in lymphoblastoid cells from autistic children and which improved following treatment with the glutathione precursor N-acetylcysteine.12 Mitochondrial dysfunction in autism has been suggested as a contributor to the generation of an oxidized microenvironment.41 In this study, we measured nuclear respiratory factor 2 (NRF-2) in the serum of autistics and control subjects. This transcription factor of the Ets family is important in controlling mitochondrial bioenergetics being required for the expression of a number of nuclear-encoded mitochondrial proteins including Tfam or the specific mitochondrial transcription factor.24,42 NRF-2 comprises NRF-2α subunit that binds DNA and NRF-2β, the transcription activation subunit.43 Deletion of the DNA binding component of NRF-2 has been found to result in reduced mitochondrial mass, ATP production and oxygen consumption as well as mitochondrial protein synthesis.23 The findings in the present study indicated significant increase in serum NRF-2 in autistic children, suggesting compensatory upregulation of this transcription factor in face of decreased mitochondrial function.
The Bcl2 family of proteins controls the mitochondrial pathway of apoptosis or programmed cell death.44 The Bcl2 family comprises the apoptotic proteins Bax (Bcl2-associated X protein) and Bak (Bcl2 antagonist/killer) and antiapoptotic proteins including Bcl2 itself. In response to apoptotic signals, Bax translocates to the outer mitochondrial membrane and together with Bak induces permeabilization of the membrane. This is followed by the release of cytochrome c into the cytosol and the consequent activation of the apoptotic pathway. Bcl2 precludes the proapoptotic activity of activated Bax and Bak.25 In this study, an increase in the level of the antiapoptotic factor -cell leukemia/lymphoma 2 (Bcl2) was observed in the serum of autistics. Other researchers reported decreased Bcl2 expression in the in the autistic brain and in lymphoblasts from autistic subjects.45-47 Bcl2 expression is sensitive to oxidative stress and decreased expression is found in hippocampal neuronal cells exposed to hydrogen peroxide (H2O2).48 On the other hand, Bcl2 overexpression confers cell resistance to oxidants such as H2O2 and superoxide anion radical (O2•−).49 Bcl-2 affects cellular levels of antioxidants50,51 and Bcl-2-deficient mice showed increased oxidative stress and vulnerability to oxidants.50 It is thus suggested that upregulation of Bcl2 in serum of autistic children observed in the current study might represent a response to the elevated levels of oxidative stress which has been shown in these subjects1.1-13
Cholinesterases catalyze the hydrolysis of the neurotransmitter acetylcholine (ACh) terminating its action at cholinergic sites in the nervous systems i.e., the neuronal synapses in the central nervous system, the neuromuscular junction, the autonomic ganglia and the post-ganglionic parasympathetic nerve fibers at innervated organs. Both acetylcholinesterase (AChE, (E.C. 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8), also known as pseudocholinesterase plasma cholinesterase, hydrolyze acetylcholine but with differing substrate specificity that is AChE is faster in hydrolyzing acetylcholine than other choline esters while BChE hydrolyzes butyrylcholine more rapidly.52,53 Cholinergic neurotransmission is important for cognitive functioning and centrally acting AChE inhibitors are in use in subjects with Alzheimer’s disease and there is also an evidence to suggest a benefit from inhibiting BChE.54,55 Studies suggested alteration in brain cholinergic system in autism.18,56 Nicotinic receptor abnormalities in the cerebral cortex and cerebellum of autistics were reported.56,57 Using positron emission tomography, Suzuki et al.18 detected decreased hydrolytic activity of AChE in the fusiform gyrus, and suggested a deficit in presynaptic cholinergic innervations in adults with autism. On the other hand, AChE inhibitors eg., rivastigmine and donepezil have been attempted in autism to improve the deficient executive function but with varied results.58,59 In practice, measuring plasma and serum BChE is a sensitive indicator of exposure to organophosphorus insecticides for inhibition of cholinesterase activity is the main mechanism of their toxicity.60 BChE might also be a useful marker of inflammation since increased serum activity was found in patients with hyperlipidaemia61 or with the metabolic syndrome.62 Moreover, BChE activities in serum as well as AChE activities in lymphocytes and whole blood increase in the relapsing-remitting form of multiple sclerosis. This occurred along with marked increments in the pro-inflammatory cytokines interferon-g (INF-g), INF- α, interleukin-1 (IL-1) and IL-6 in serum.63 In this study, we measured serum cholinesterase (BChE) activity in children and adolescents affected with autism. A markedly increased BChE activity was found in the serum of autistic patients compared to their controls. The significance of this finding is yet to be determined. This increase in cholinesterase activity implies decreased cholinergic tone which might have a role in the increased inflammatory response observed in autism. Serum BChE might also serve as a biomarker for autistic disorders.
Conflicts of Interest
The authors declare that there are no potential conflicts of interest.
Acknowledgements
This works is was not supported by research grants
References
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2016;3:1-21
- Gerberding J. Prevalence of autismspectrum disorder–Autism and developmental disabilities monitoring network, six sites, United States, 2000 and2002. MMWR Morb Mortal Wkly Rep. 2007;56:1 -40.
- Fombonne E., Zakarian R., Bennett A., Meng L., McLean-Heywood D. Pervasive developmental disorders inMontreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118:e139 -150.
CrossRef - Bhat S., Acharya U. R., Adeli H., Bairy G. M., Adeli A. Autism: cause factors, early diagnosis and therapies. Rev Neurosci. 2014;25:841-850.
CrossRef - Muhle R., Trentacoste S. V., Rapin I. The genetics of autism. Pediatrics. 2004;113:e472-486.
CrossRef - AL-Ayadhi L. Y., Mostafa G. A. Serum antinucleosome-specific antibody as a marker of autoimmunity in children with autism. J Neuroinflammation. 2014;11:69.
CrossRef - Theoharides T. C. Is a subtype of autism an allergy of the brain? Clin Ther. 2013;35:584-591.
CrossRef - Vojdani A., Pangborn J. B., Vojdani E., Cooper E. L. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol. 2003;16:189-199.
CrossRef - Vojdani A., O’Bryan T., Green J. A., Mccandless J., Woeller K. N., Vojdani E., Nourian A. A., Cooper E. L. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr Neurosci. 2004;7:151-161
CrossRef - Mostafa G. A., El-Sayed Z. A., El-Aziz M. M., El-Sayed M. F. Serum anti-myelin-associated glycoprotein antibodies in Egyptian autistic children. J Child Neurol. 2008;23:1413-1418.
CrossRef - László A., Novák Z., Szőllősi-Varga I., Hai du Q., Vetró Á., Kovács A. Blood lipid peroxidation, antioxidant enzyme activities and hemorheological changes in autistic children. Ideggyogy Sz. 2013;66:23-28
- Rose S., Frye R. E., Slattery J., Wynne R., Tippett M., Melnyk S., James S. J. Oxidative stress induces mitochondrial dysfunction in a subset of autistic lymphoblastoid cell lines. Transl Psychiatry. 2014;4:e377.
CrossRef - Abdel-Salam O. M. E., Youness E. R., Mohammed N. A., Abu Elhamed W. A. Nuclear Factor-Kappa B and Other Oxidative Stress Biomarkers in Serum of Autistic Children. Open J Mol Integr Physiol. 2015;5:18-27 .
CrossRef - Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I. N., Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196-199.
CrossRef - Theoharides T. C., Tsilioni I., Patel A. B., Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry. 2016;6:e844.
CrossRef - Shen Y., Ou J., Liu M., Shi L., Li Y., Xiao L., Dong H., Zhang F., Xia K., Zhao J. Altered plasma levels of chemokines in autism and their association with social behaviors. Psychiatry Res. 2016;244:300-305.
CrossRef - Cohly H. H., Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
CrossRef - Suzuki K., Sugihara G., Ouchi Y., Nakamura K., Tsujii M., Futatsubashi M., Iwata Y., Tsuchiya K. J., Matsumoto K., Takebayashi K., Wakuda T., Yoshihara Y., Suda S., Kikuchi M., Takei N., Sugiyama T., Irie T., Mori N. Reduced acetylcholinesterase activity in the fusiform gyrus in adults with autism spectrum disorders. Arch Gen Psychiatry. 2011;68:306-313.
CrossRef - Gargus J. J and Imtiaz F. Mitochondrial energy-defi cient endophenotype in autism. Am J Biochem Biotechnol. Special Issue on Autism Spectrum Disorders. 2008;4:198–207.
- Napoli E., Wong S., Hertz-Picciotto I., Giulivi C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics. 2014;133:e1405-1410.
CrossRef - Sparks B. F., Friedman S. D., Shaw D. W., Aylward E. H., Echelard D., Artru A. A., Maravilla K. R., Giedd J. N., Munson J., Dawson G., Dager S. R. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184-192.
CrossRef - DeRamus T. P., Kana R. K. Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. Neuroimage Clin. 2014;7:525-536.
CrossRef - Yang Z. F., Drumea K., Mott S., Wang J., Rosmarin A. G. GABP transcription factor (nuclear respiratory factor 2) is required for mitochondrial biogenesis. Mol Cell Biol. 2014;34:3194-3201.
CrossRef - Priya A., Johar K., Wong-Riley M. T. Nuclear respiratory factor 2 regulates the expression of the same NMDA receptor subunit genes as NRF-1: both factors act by a concurrent and parallel mechanism to couple energy metabolism and synaptic transmission. Biochim Biophys Acta. 2013;1833(1):48-58.
CrossRef - Czabotar P. E., Lessene G., Strasser A., Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15:49-63 (2014).
CrossRef - American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV. 4th Edition, American Psychiatric Association, Washington DC (1999).
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab 23:748-755 (2003).
CrossRef - Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2_/_ mice. J Cereb Blood Flow Metab 30:769–782 (2010).
CrossRef - Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57: 67–81 (2005).
CrossRef - Han YM, Cheung WK, Wong CK, Sze SL, Cheng TW, Yeung MK, Chan AS. Distinct Cytokine and Chemokine Profiles in Autism Spectrum Disorders. Front Immunol 8:11 (2017).
CrossRef - Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol 21:279-297 (2011).
CrossRef - Thompson WL, Karpus WJ, Van Eldik LJ. MCP-1-deficient mice show reduced neuroinflammatory responses and increased peripheral inflam¬matory responses to peripheral endotoxin insult. J Neuroinflammation 5:35 (2008).
CrossRef - Losy J, Zaremba J. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke 32:2695-2696 (2001).
CrossRef - Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm 2005:175-179 (2005).
CrossRef - Van Der Voorn P, Tekstra J, Beelen RHJ, Tensen CP, Van Der Valk P, De Groot CJA. Expression of MCP-1 by Reactive Astrocytes in Demyelinating Multiple Sclerosis Lesions. Am J Pathol 1999; 154(1): 45–51.
CrossRef - Legido A, Jethva R, Goldenthal MJ. Mitochondrial dysfunction in autism. Semin Pediatr Neurol 20:163-175 (2013).
CrossRef - Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: An update and review. Biochimica et Biophysica Acta 1757: 509–517 (2006).
CrossRef - James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, Gaylor DW. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J 23: 2374–2383 (2009).
CrossRef - Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med 66:36-44 (2014).
CrossRef - Ma Q. Role of Nrf2 in Oxidative Stress and Toxicity. Ann Rev Pharmacol Toxicol 53: 401-426 (2013).
CrossRef - Frye RE, Delatorre R, Taylor H, Slattery J, Melnyk S, Chowdhury N, James SJ. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl Psychiatry 3:e273 (2013).
CrossRef - Bruni F, Polosa PL, Gadaleta MN, Cantatore P, Roberti M. Nuclear Respiratory Factor 2 Induces the Expression of Many but Not All Human Proteins Acting in Mitochondrial DNA Transcription and Replication. J Biol Chem 285:3939–3948 (2010).
CrossRef - Hayashi R, Takeuchi N, Ueda T. Nuclear Respiratory Factor 2β (NRF-2β) recruits NRF-2α to the nucleus by binding to importin-α:β via an unusual monopartite-type nuclear localization signal. J Mol Biol 425:3536-3548(2013).
CrossRef - Wong WW, Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life 60:390-397 (2008).
CrossRef - Araghi-Niknam M, Fatemi SH. Levels of Bcl-2 and P53 are altered in superior frontal and cerebellar cortices of autistic subjects. Cell Mol Neurobiol 23:945-952 (2003).
CrossRef - Sheikh AM, Li X, Wen G, Tauqeer Z, Brown WT, Malik M. Cathepsin D and apoptosis related proteins are elevated in the brain of autistic subjects. Neuroscience 165:363-370 (2010).
CrossRef - Malik M, Sheikh AM, Wen G, Spivack W, Brown WT, Li X. Expression of inflammatory cytokines, Bcl2 and cathepsin D are altered in lymphoblasts of autistic subjects. Immunobiology 216:80-85 (2011).
CrossRef - Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem 84:982-996 (2003).
CrossRef - Amstad PA, Liu H, Ichimiya M, Berezesky IK, Trump BF, Buhimschi IA, Gutierrez PL. BCL-2 is involved in preventing oxidant-induced cell death and in decreasing oxygen radical production. Redox Rep 6:351-362 (2001).
CrossRef - Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J Neurochem 71:741-748 (1998).
CrossRef - Jang JH, Surh YJ. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-kappaB activation. J Biol Chem 279:38779-86 (2004).
CrossRef - Silman I, Sussman JL. Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol 5:293-302 (2005).
CrossRef - Çokuğraş AN. Butyrylcholinesterase: Structure and Physiological Importance. Turk J Biochem 28; 54-61 (2003).
- Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 41:615-631 (2014).
CrossRef - Giacobini E. Selective inhibitors of butyrylcholinesterase: a valid alternative for therapy of Alzheimer’s disease? Drugs Aging 18: 891–898 (2001).
CrossRef - Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, Iversen P, Bauman M, Perry E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 125:1483-1495 (2002).
CrossRef - Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH, Wenk GL. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry 158:1058-1066 (2001).
CrossRef - Chez MG, Aimonovitch M, Buchanan T, Mrazek S, Tremb RJ. Treating autistic spectrum disorders in children: utility of the cholinesterase inhibitor rivastigmine tartrate. J Child Neurol 19:165-169 (2004).
- Handen BL, Johnson CR, McAuliffe-Bellin S, Murray PJ, Hardan AY. Safety and efficacy of donepezil in children and adolescents with autism: neuropsychological measures. J Child Adolesc Psychopharmacol 21:43-50 (2011).
CrossRef - Eddleston M, Chowdhury FR. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br J Clin Pharmacol 81:462–470 (2016).
CrossRef - Kálmán J, Juhász A, Rakonczay Z, Abrahám G, Zana M, Boda K, Farkas T, Penke B, Janka Z. Increased serum butyrylcholinesterase activity in type IIb hyperlipidaemic patients. Life Sci 75:1195-1204 (2004).
CrossRef - Randell EW, Mathews MS, Zhang H, Seraj JS, Sun G. Relationship between serum butyrylcholinesterase and the metabolic syndrome. Clin Biochem 38:799-805 (2005).
CrossRef - Polachini CR, Spanevello RM, Casali EA, Zanini D, Pereira LB, Martins CC, Baldissareli J, Cardoso AM, Duarte MF, da Costa P, Prado AL, Schetinger MR, Morsch VM. Alterations in the cholinesterase and adenosine deaminase activities and inflammation biomarker levels in patients with multiple sclerosis. Neuroscience 266:266-274 (2014).
CrossRef








