Nazar Sh. Mohammed1, Fadia Abd Al-Muhsin. Al-khayat2 and Saad Kh. Hussien3
1Department of Technical Analytical/ College of Health and Medical Technology / Middle Technical University/Baghdad /Iraq MTU
2Department of Basic sciences/ College of Dentistry / Baghdad university/Baghdad /Iraq.
3Department of Nursing/ Medical-Technical Institute/ Middle Technical University/Baquba/Iraq MTU.
Corresponding Author E-mail: nazarnazar909@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1135
Abstract
Toxoplasmosis is the most common, widespread disease in the world which is caused by Toxoplasma gondii.The objective of the current study is to determine the effect of the Toxoplasma gondii infection on male sperm, especially on the mitochondria of sperm for men who suffer infertility and the possibility of a hereditary mutation. Sixty seminal fluid and serum samples were taken from sub- fertile patients who attended Teba center for in vitro fertilization / Babylon and similarly samples were also obtained from healthy individuals as a control group, their ages ranged from 20 to 60 years old during the period from 1st may /2016 till 25th January/2017. All samples subjected to the tests included Macroscopic and microscopic examination, molecular methods was used for DNA extraction, amplification and sequencing. Statistical analysis showed non-significant differences (P<0.01) among age groups and ) between rural 41(68%) and urban 19(32%). However, sperm motility and count recorded highly significant differences (P<0.01) in the mean value for healthy (74.85, 68.017) as compared with the patients (10.30, 10.217). There were also a significant differences (P<0.01) in the mean concentration of Ant-Toxoplasma IgG antibody (1.327µ/ml) in comparison with control group (0.564µ/ml). A highly significant differences (P<0.01) was detected when comparing the positive 55(92%) and negative 5(8%) samples for oligospermia and a significant difference (P<0.05) for Asthenospermia 21(35%), 39(65%). Two mutation 2(3%) were detected in the exchange for fifty eight 58(97%) with a highly significant difference (P<0.01). It was found that the mutation occurred in Asthenospermia of ND1 gene in the site SNP T4216C (T to C) and the site SNP C3450T (C to T) of Mitochondrial DNA. This study confirmed that a mutation in the ND1 gene that is located in the mitochondria of sperms, specifically in people who suffer from infertility, is occurred due to Toxoplasma gondii.
Keywords
Toxoplasma gondii; Molecular methods; genetic mutation
Download this article as:| Copy the following to cite this article: Mohammed N. S, Al-Muhsin. Al-khayat F. A, Hussien S. K. The Impact of Toxoplasma Gondii on Mitochondrial DNA of Sub-Fertile Men Sperms. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Mohammed N. S, Al-Muhsin. Al-khayat F. A, Hussien S. K. The Impact of Toxoplasma Gondii on Mitochondrial DNA of Sub-Fertile Men Sperms. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=14915 |
Introduction
Toxoplasmosis is the most common, widespread disease in the world which is caused by Toxoplasma gondii. This parasite is transmitted by several ways, and recent studies have proven that this parasite is possible to be carcinogenic (1). Domestic and wild cats which belong to the Feline family consider to be as definitive host while, warm-blooded animals including human represent as intermediate host. Human toxoplasmosis classified as food and water born disease, generally occurs through consuming the infective stage oocysts or tissue cysts containing the bradyzoites form. Also transmission by organ transplantation and through placenta ( from infected mother to fetus) were documented as routes of infection (2). Some clinical studies have documented that T. gondii affect reproductive parameters of men , recent studies have demonstrated the transmission of the disease through sexual contact from the husband to his wife and vice versa (3). As similar studies have proven that sexual transmission has satisfactory complications including infertility (4). Some studies have indicated that this parasite works on abnormalities in the mitochondria, of sperm, causing their death, thus infertility occurs in people with T. gondii infections (5).
In the current work, the effect of toxoplasmosis on reproductive system in men with the effect of genetic mutation of sperms in the infertility incidence was studied for the first time in Iraq. The mutation velocity of mitochondrion of sub-fertile men DNA has been expected to be 10 fold more than that of nuclear DNA (5, 6) because the presence of mutations is associated with a diversity of human being diseases (7, 8), reliability of mt DNA duplication, appear to lack a competent of DNA renovate system compared with the nuclear DNA repetition system (9)and also lacks safety of histone protein. Therefore, mutations may reason from DNA damage caused by peripheral factors (10). Abnormal sperm has a direct effect on pregnancy rates. Here’s an overview of how azoospermia, oligospermia, and asthenospermia and other sperm health problems affect pregnancy. The low number of sperms is considered one of the most important reasons leading to fertility issues In couples who have difficulty getting pregnant, Guo J. et al,(11).
Materials and Methods
Patients and Control Group
Sixty samples for each seminal fluid and sera of both healthy individuals and patients of sub- fertile men were included in this study and whose ages range from 20-60 years old who attended TEBA center for children / Babylon during the period from the beginning of may / 2016 to the last of January/2017. Patients were found to be suffering from infertility either with Asthenospermia, or oligospermia.
Seminal Fluids Analysis
Sample Collection
The samples were collected after a minimum of (3-5) days of sexual intercourse. Samples were kept from temperature changes until they reached the laboratory (20°C > save samples >40°C).
Serum Samples
For the purpose of measuring the concentration of Anti- Toxoplasma IgG in the serum of both healthy and patients. Two to three ml was collected in sterile tubes and kept in the freeze until use.
Macroscopic Examination
Liquefaction
The sample of the seminal fluid is liquidated at room temperature within half an hour, although the liquefaction usually takes about a quarter of an hour.
Volume
For the purpose of measuring the size of ejaculation samples, a cone-based cylinder was used.
Viscosity
The viscosity (sometimes referred to as “consistency”) of the liquefied sample should be recognized as being different from coagulation.
pH
For the purpose of measuring pH, a drop of semen was placed on the pH paper (range: 6.1 ≤ pH ≥10.0 or 6.4 ≤ pH ≥ 8.0).
Microscopic Examination
Preparation of semen samples
After liquefaction, one drop of 10μl of homogenized semen was placed on a warm clean microscopic glass slide with a micropipette and covered with a cover slip of 22mm X22 mm.
Sperm Count
the count of sperms ( how many million sperms are there in one ml of each seminal fluid samples) was estimated by calculating the mean number of spermatozoa in 10 random microscopic fields multiplied by million (106).
Percentage of Motility and Degree of Activity For Sperms
Ten random fields were examined and the percentage of moving sperm was calculated, by using equation:
Motility =Number of motile sperm in ṉ (number) of fields multiplied by 100 divided by total number of sperm in ṉ fields.
DNA analysis of Mitochondria (mt DNA)
Extraction and Amplification of Mitochondrial DNA
Mitochondrial isolation kit ( Biovision) was used for mt DNA extraction . Agarose gel electrophoresis (1.2%) was used for visualization of extracted mt DNA as gel loading buffer was prepared by mixing three µl of the extracted DNA with 3µl of Orange G (30% glycerol and 35% Orange G). Electrophoresis was done using 1x TBE buffer at 5 V/cm for half an hour. Gel were stained with ethidium bromide (half a gram/ml) and then visualized by using an Ultra- Violate trans illuminator (300 nm).
The UV spectrophotometer device was used to determine the concentration and purity of the extracted DNA. The concentration was calculated using absorbance at 260 nm reading (one optical density unit) which corresponds to 50 µg/ml DNA.
PCR Reaction and Application
Two sets of primers were designed, for ND1 mitochondrial genes amplification. The reactions of PCR were optimized for each primer pair with different thermal grades used for annealing process. Approximately 160 ng of total sperm DNA was used in 50 μL of reaction mixture. The PCR reaction mixture contained the following:
1.5 μmMg+2
200 μm of deoxynucleotide triphosphate (dATP, dCTP, dTTP, dGTP)
1 × Taq buffer, 0.6 μm of each primer and
1 unit of Taq DNA polymerase (Roche).
During PCR cycle, temperature 94°C was used for two minutes in the initial denaturation process, followed by 35 cycles of denaturing within the same temperature but for 30 second , annealing was done at 58°C for 45 second and extension of primers was carried out at 72 C0 for 2 min. while the final extension required 5 min. at 72°C .The reaction products were saved at 4°C.
The primers for the ND1 gene are
F 5′-ACATACCCATGGCCAACCTC-3′ and R 5′-AATGATGGCTAGGGTGACTT-3′.
5′-ATGTTTGAGACCTTCAACAC-3′ and 5-CATCTCTTGCACGAAGTCGA-3′ respectively.
DNA Sequencing
Automated mt DNA sequencing of the ND1 gene was performed for different fertility groups using the Bio- Applied System, dye TM termination V 3.1 cycle sequencing kit.
Evaluation of DNA Sequencing
All the data obtained from automated sequence was edited with a computer based program sequencer TM. To determine the nature of mutations, compare with the reference sequence (NCBI database, accession number: NC_00187) has been adopted.
Analysis for Silent Mutations
The synonymous mutations were analyzed for codon usage. Data obtained from this study and data from Bail and Bebok 2016(12) were analyzed for identification of amino acid changes by the computer program MEGA version 21 (13).
Analysis for Non-Silent Mutations
Hydrophobicity and polarity were used in protein analysis for the purpose of determining the effect of amino acid changes on protein properties , this was done with computer based program (http://www.roselab.jhu.edu/ ~raj/ MISC/ hphobh.html) and ProtScal (http://download.invitrogen.com/evergreen /support_files/a-protscale-expasy.htm)
Statistical Analysis
The program SPSS version 16 and Microsoft Office Excel 2007 were used for data analysis. Numeric data were expressed as mean ± standard error of mean (SEM ). Unpaired t-test and chi squared was used to compare numeric data for healthy control and patients groups while Chi-square was used to assess the differences between the proportions. P-value <0.05 was considered significant.
Results
1-The distribution of Toxoplasma infection among studied groups according to the age and the residency
In table one , the demographical picture for the age group showed that from the sixty total samples 24 of healthy men (40%) and 21 of patients (35%) were aged between 20-40 years old while, 36 (60%) and 39(65%) were aged between 41-60 years old for healthy and patients respectively. There were no significant differences (P<0.05) when comparing the two groups. According to residency, no significant differences (P<0.05) was recorded when compared between rural 39(65%), 41(68.3%) and urban areas 21(35%),19(32.7%), for healthy and patients respectively.
Table 1: Distribution of Toxoplasma infection among studied groups according to the age and the residency:
| Parameters | Studied groups | Chi-Square
Test (P-value) |
||||
| Healthy
(N= 60) |
Patients ( (N= 60) |
Total (N=120) |
||||
| Age groups
/ Year |
20 – 40 | N | 24 | 21 | 45 | X2 value=0.32
P=0.572
|
| % | 40% | 35% | ||||
| 41 – 60 | N | 36 | 39 | 75 | ||
| % | 60% | 65% | ||||
| Residency | Rural | N | 39 | 41 | 80 | X2 value=0.15
P = 0.699
|
| % | 65% | 68.3% | ||||
| Urban | N | 21 | 19 | 40 | ||
| % | 35% | 31.7% | ||||
According to the results in table two, highly significant differences (P<0.01) were observed in both sperm motility and count when comparing two groups. The mean of sperm motility and sperm count was 74.85, 10.30 and 68.017, 10.217 for healthy and patients respectively.
Table 2: Sperm motility and count in healthy and patients samples
| Parameters | Test groups | No. | Mean | TD | S.E | t-test |
| (P-value) | ||||||
| Sperm | healthy | 60 | 74.85 | 11.663 | 1.506 | P <0.01 |
| motility% | Patients | 60 | 10.3 | 7.021 | 0.906 | |
| Total | 120 | |||||
| Sperm count | healthy | 60 | 68.017 | 11.199 | 1.4457 | P <0.01 |
| million/ml | Patients | 60 | 10.217 | 5.6869 | 0.7342 | |
| Total | 120 |
Demographical Picture of Anti- Toxoplasma antibodies IgG, among the studied Groups
Results in table three revealed a highly significant difference (P<0.01) in the concentration of IgG antibodies of patients group in contrast with healthy group. The mean concentration of IgG was 0.564 µ /ml for healthy and 1.327 µ /ml for patients.
Table 3: The concentration of IgG antibody in serum among studied groups
| Parameters | Test groups | No. | Mean | S.D | S.E | P |
| µ /ml | ||||||
| Serum Anti– | healthy | 60 | 0.564 | 0.201 | 0.026 | < 0.01 |
| Toxoplasmosis | Patients | 60 | 1.327 | 0.584 | 0.076 | |
| IgG Ab. | Total | 120 |
Positive and negative cases of Oligospermia, Asthenospermia, and mutation in seminal fluids among sub-fertile patients
Table four elucidate that for both of oligospermia sperm and mutation in ND1 gene of mitochondrial DNA, there was a highly significant differences (P<0.01) between positive 55(92%), 2(35%) and negative 5(8%), 58(97%) sub-fertile patients respectively. While, the Asthenospermia showed significant difference (P<0.05) between positive and negative 21(35%) , 39(65%) respectively.
Table 4: The positive and negative numbers of abnormal cases in seminal fluid of sub-fertile patients
| Parameters for | No. | Percentage | P | |
| Patients | % | |||
| Oligospermia | +ve | 55 | 92% | < 0.001 |
| -ve | 5 | 8% | ||
| Total | 60 | 100% | ||
| Asthenospermia | +ve | 21 | 35% | 0.027 |
| -ve | 39 | 65% | ||
| Total | 60 | 100% | ||
| Mutation in ND1 gene of mt DNA | +ve | 2 | 3% | <0.001 |
| -ve | 58 | 97% | ||
| Total | 60 | 100% | ||
Amplification of mt DNA
PCR reactions were performed to amplify the ND1 genes from the extracted DNA samples. Among them, twenty-one sample showed the appropriate product size of the DNA (1183 bp) with ND1 were digested with restricted enzymes, EcoRI and HindIII. Figure (1) shows ND1 PCR products for 5 samples.
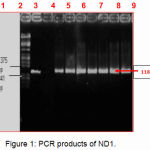 |
Figure 1: PCR products of ND1.
|
Lane M
Lambda DNA marker digested with restricted enzymes, EcoRI and HindIII.
lane 1
Positive control (sample containing extracted DNA of correct PCR product size for ND1).
Lane 2
Negative control (sample without extracted DNA).
Lane 3 4,5: 6,7,8 and 9
Asthenozoospermic Electrophoresis was done on an agarose gel (1.2%) using 1 × TBE buffer and visualized by using an ultra-violet (UV) transilluminator (300 nm).
DNA Sequence Analysis: (ND1 gene)
After purification of PCR products, automated DNA sequencing was performed using the Applied Bio- System Big Dye TM termination V 3.1 cycle sequencing kit. Twenty one of sub- fertility samples were sequenced for the ND1 gene (14). These sequences were compared with the reference value obtained from the National center for biotechnology information (NCBI) database. A computer software program Sequencer TM was used to edit the gene sequences and for determination the nature of mutations. Two mutations in different nucleotide position in ND1 region were found in different groups of the samples. The base substitutions were located at nucleotides (nts) 3450, 4216 (Table 4).
Single Nucleotide Polymorphisms (SNP) T4216C
The evolution of T to C was notorious at nt 4216 in the ND1 gene. This SNP was found in an Asthenospermia of man (sample code, 010480). This SNP is an equivalent changeover in the third position of a tyrosine codon, altering it from TAC to TAT at nt 3480 (Figure 2, Table 4).
Single Nucleotide Polymorphisms (SNP) C3450T
The evolution of C to T transition was identified at nt 3450 in ND1 region. This transition was observed in an asthenozoospermic (code 6445) sample. This SNP, a synonymous substitution occurred in the third position of the proline codon, changing the codon CCC to CCT (Table 4).
Analysis of Codon Usage in Synonymous Mutants in Mitochondrial DNA
The frequency of codon usage for each mitochondrial gene was determined using the computer program MEGA version 6.06, (13). The amino acid and their frequency change rate were determined. Analyses of codon usage in synonymous mutants between fertile and Asthenospermia are tabulated in Table 4.
Table 4: DNA sequence analysis and the codon usage of synonymous mutations of semen samples in mitochondrial DNA.
| Cases | Gene | Mutation | Codon change | Amino Acid
Change |
Codon Frequency
Change |
| Asthenospermia | ND1 | T4216C* | TAT-TAC | Silent | 1.6-1.63 |
| Asthenospermia | ND1 | C3450T* | CCC-CCT | Silent | 1.04- .087 |
* indicates the mutations obtained from different groups of semen samples
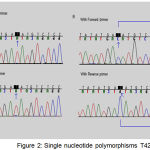 |
Figure 2: Single nucleotide polymorphisms T4216C
|
Statistical Analysis for Gene Sequencing
A statistical analysis was performed on individual SNPs that were identified in at least 2 samples of the total fertile and sub-fertile population. The Z value (binomial test) was determined for testing of significant differences between the mutants/polymorphisms in the sets of fertile and sub-fertile men. The Z value was calculated from Z = | P1 – P2 | / √ (P (1-P)/n) (15).
Discussion
This study was conducted for the first time in Iraq, to study the possibility of the effect of toxoplasmosis on infertility of the men. The results revealed a non- significant differences between the age groups (42.55±6.753) in comparison with control group (40.37±7.863) among the toxoplasmosis patients, this is in line with the finding of many studies such as (Mohammed 2016) (1) and (Singh, 2016) (2). Non- significant difference was recorded between rural 41(68%) and urban 19(32%) according to residency this result is in agreement with (Singh, 2016) (2). The result also demonstrated that there were highly significant differences (P<0.01) regarding to the elevation of the concentration of Ant-Toxoplasma IgG antibody (1.327±0.564) in comparison with control group (0.564± 0.201) among studied groups. This is consistent with Thamm et al, 2016, (16) who identified that by using application of multivariate regression has increased of seroprevalence from 20% (95%-CI:17–23%) in the 18–29 age group to 77% (95%-CI:73–81%) in the 70–79 age group (16), these results also agreed with Ollivier et al, 2016 (17), and Islamorada, 2013, whose studies showed that chronic toxoplasmosis affected on reproductive parameters in men, (18). Nevertheless, there was a significant increase between the census of sperm count and the motility of sperm in similitude with control groups (10.217± 5.6869), (10.30±7.021), (68.017±11.1985). (74.85±11.663), respectively between patients with toxoplasmosis infection. These results are congruence with Dalimi, 2013 (6), who demonstrated that toxoplasmosis was highly impact on the production of sperm and its vitality and effectiveness, (6). Shiadeh, et al,2016, proved in a study that Toxoplasma gondii could cause endometritis, impaired folliculogenesis, ovarian and uterine atrophy, adrenal hypertrophy, vasculitis, and cessation of estrus cycling in female and in male cause decrease in semen quality, concentration, and motility (19). While Ab-dulla, et al, reported that during infection, each of the parasite and the host used various mechanisms to enhance their own reproductive success, along with an ever-growing number of couples suffering from idiopathic infertility. Although there is a lack of research on how T. gondii can alter reproductive parameters, one of the common methods for determination of fertility status in males is seminal fluid analysis (4). In the present work, the results showed that a significant differences for each of oligospermia, 55 (92%) were positive and 5 (8%) were negative that a highly significant difference (P<0.01), while the Asthenospermia 21(35%) and 39(65%), with a significant difference (P<0.05) and mutation were 2(35%) were positive and 58(97%) highly significant (P<0.01). This study was performed for first time in Iraq, and has proven that there was a genetic mutation in the base substitutions and that there was a change in SNP T4216C position, T to C transition was identified at nt 3396 in the ND1 gene. This SNP was found in an Asthenospermia man (sample code 010573), and replace synonym in the third position of a tyrosine codon, changing it from TAT to TAC, Another SNP was found at C3450T position, C to T transition was identified at nt 3450 in ND1 region. This transition was observed in an asthenozoospermic (code 6445) sample and replace synonym in the third position of the proline codon, changing the codon from CCC to CCT, these results were obtained using the analysis of codon usage in synonymous mutants in mitochondrial mt DNA (13). Other studies reported obtaining genetic mutation that assigned the current study. Whereas these results were in a harmony with Hoang, et al, (20) they revealed that mutation from either exogenous or endogenous sources, like DNA replication errors or environmental insults like smoking or sunlight and they developed the bottleneck sequencing system (BotSeqS) as a simple genome-wide sequencing-based method that accurately quantitates nuclear and mitochondrial mutational load in normal human tissues also, they demonstrated that mutation prevalence and spectrum vary depending on age, tissue type, DNA repair capacity, and carcinogen exposure. These results suggested a varied landscape of rare mutations within the human body that has yet to be explored (19). Also there is convergence with Chen et al, (2016) (21), who found that a comprehensive screen of 874 genes in 589,306 genomes led to the identification of 13 adults harboring mutations for 8 severe Mendelian conditions, with no clinical manifestation of the indicated disease. Our findings demonstrate the promise of broadening genetic studies to systematically search for well individuals who are buffering the effects of rare, highly penetrant, deleterious mutations (21). Therefore, this study was conducted to determine a genetic mutation in the ND1 gene that is located in the mitochondria of sperms, specifically in people who suffer from infertility, and based on these previous studies. This study demonstrated that this mutation occurred due to Toxoplasma gondii infections.
Reference
- Abd AL-Gabbar M S. Seroprevalence Determination of Toxoplasmosis among Cancer Patients teses University of Diyala, College of Education of pure science ;(2016).
- Singh S, Congenital toxoplasmosis: Clinical features, outcomes, treatment, and prevention, Trop Parasitol. 2016; 6(2): 113–122.
CrossRef - Flegr J, Klapilová K and Kanˇková Š. Toxoplasmosis can be a sexually transmitted infection with serious clinical consequences. Not all routes of infection are created equal. Medical Hypotheses J. 2014; 83: 286–289.
CrossRef - Husam E. Ab-dulla , Ahmed S. Abood, Haider S. Kadhim, Nada M. Al-bashier and Ula Al-kawaz. The impact of toxoplasmosis on Seminal fluid analysis in association with infertility. Inter J of Scientific & Engin Research .2016;7(5):70-73
- Vazharova R and Kremensky Individual capacity for DNA repair and maintenance of genomic integrity: a fertile ground for studies in the field of assisted reproduction, J. Biotechnology & Biotechnological Equipment 2016 ;30(3): 419-433.
CrossRef - Dalimi A and Abdoli Toxoplasma gondii and Male Reproduction Impairment: A new Aspect of Toxoplasmosis Research Jundishapur Journal of Microbiology. 2013 ; 6(8): e7184.
- Wilking H, Thamm M and Stark K, Aebischer T and Seeber F . Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. J Sci Rep. 2016; 6: 22551.
CrossRef - Colosi H A , Jalali-Zadeh B and Colosi I A ,Simon L M and Costache C A. Influence of Toxoplasma gondii Infection on Male Fertility: A Pilot Study on Immunocompetent Human Volunteers Iran J Parasitol. 2015; 10(3): 402–409.
- János M ,Gergely S and Gábor E T. Characterization of Disease-Associated Mutations in Human Transmembrane Proteins, PLoS One. 2016; 11(3): e 0151760.
- Bing Yu,Paul S. de Vries , Ginger A M, Zhe W, ElenaV F, Xiaoming L, Donna M M, Lynne E W, Richard A G,Alanna C M and Eric B . Whole genome sequence analysis of serum amino acid levels. Genome Biology 2016;17:237.
CrossRef - Guo J, Wang F,Zhang Q, Geng Q, Yu G J, Zhao J Y, Gao Q H and Song CS. Treatment of oligospermia/asthenozoospermia patients by three different Chinese medical principles: a randomized control clinical study National Institutes of Health 2013;33(9):1170-1173.
- Vedrana Baliand Zsuzsanna Bebok, Decoding Mechanisms by which Silent Codon Changes Influence Protein Biogenesis and Function, Int J Biochem Cell Biol. 2015 Jul; 64: 58–74.
CrossRef - Dongfang Hu, Lin Lv, Jinyuan Gu ,Tongyu C, Yihong.X and Sidang L.; Genetic Diversity and Positive Selection Analysis of Classical Swine Fever Virus Envelope Protein Gene E2 in East China under C-Strain Vaccination, Front Microbiol. 2016; 7: 85.
CrossRef - Ha Thuc Ai Hien,Tran Tan Thanh, and Nguyen Thi Mai Thu, Development and evaluation of a real‐time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma J. of Mycoses. 2016 Dec; 59(12): 773–780.
- Practically cheating statistical hand book, Z-Score: Definition, Formula and Calculation, (2016).
- Michael Thamm,Klaus Stark Toni Aebischer & Frank SeeberPrevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study, nutrent research J. doi 10.1087, 2016.
- L Ollivier H. Fricker-Hidalgo and N. Godineau et al; Serological diagnosis of Toxoplasma gondii infection 2016 Volume 84, Issue 1, Pages 22–33.
- Zahra Islamorada, Reza Haji hossein, and Behzad Ghorbanzadeh, et al; Effects of Toxoplasma gondii Infection in Level of Serum Testosterone in Males with Chronic Toxoplasmosis Iran J Parasitol. 2013 Oct; 8(4): 622–626.
- Malihe Nourollahpour Shiadeh, Maryam Niyyati Shirzad, and Fallahi Ali Rostam Human parasitic protozoan infection to infertility: a systematic review February 2016, 115, pp 469–477.
- Margaret L. Hoang, Isaac Kinde, and Cristian Tomasetti, et al; Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing :Proc Natl Acad Sci U S A. 2016 30; 113(35): 9846–9851.
- Rong Chen, Lisong Shi, and Jörg Hakenberg et al; Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood , Nature Biotechnology 34, 531–538 (2016) doi:10.1038/nbt.3514.
CrossRef








