Boddu V. S. Chandrasekhar1, Elango P1, S.Uma Maheswari2 and D. Rajukumar3
1Department of Pharmacology, Sri Ramachandra Medical College and Research Institute, Sri Ramachandra University, Porur, Chennai-600116, India.
2Department of Pharmacology, Faculty of Pharmacy, Sri Ramachandra University, Porur, Chennai – 600116, India.
3Department of Physiology, Rajah Muthiah Medical College, Annamalai University, Chidambaram, Tamil Nadu, India.
Corresponding Author E-mail : drpelango@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1199
Abstract
A long term stressful event in life altering immune system is a major factor for the development of psychopathological conditions. By now it’s clear that stress induced neuroinflammatory cytokines are the key role in mediating neurochemical and neuroendocrine systems causing the behavioral disorders and cognitive impairment. Current anti depressants are unable to correct neurotransmitters levels directly or indirectly by inhibiting cytokines expression. Present study was conducted to elucidate the effect and mechanism of L-theanine on chronic restrainer stress (CRS) induced neuroinflammation, anxiety-like, depression, learning and memory impairment in C57BL/J male mice. Chronic restrain stress was induced by well ventilated 50ml conical tube for 6 hours/day 21 consecutive days. After stressor cessation behavioral and recognition tests were conducted, at the end of study mice were sacrificed, brain and blood was collected. Cytokines (TNF-α and IL-6), neurotransmitters were quantified in prefrontal cortex and estimated corticosterone levels in plasma. Histopathological examination of the hippocampus was performed. The results was implicated that L-theanine significantly (p<0.05) reversed the effect of CRS induced behavioral changes and memory impairment, reduced cytokines expression and restored neurotransmitter levels in prefrontal cortex. It also alleviated neuronal apoptosis and lowered the corticosterone levels in plasma of CRS mice. These results indicate that L-theanine exerted protective effect on chronic stress induced neuroinflammation, anxiety, depression and cognitive dysfunction. The mechanism of protective effect is by reducing cytokine expression in frontal cortex and preventing hippocampal apoptosis.
Keywords
Stress; Neuro-inflammation; Depression; Cognition; L-theanine
Download this article as:| Copy the following to cite this article: Chandrasekhar B. V. S, Elango P, Maheswari S. U, Rajukumar D. A Focus on the Effect of L-Theanine on Improving Depression and Cognition in C57BL/J Male Mice Subjected for Chronic Stress Induced Neuroinflammation. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Chandrasekhar B. V. S, Elango P, Maheswari S. U, Rajukumar D. A Focus on the Effect of L-Theanine on Improving Depression and Cognition in C57BL/J Male Mice Subjected for Chronic Stress Induced Neuroinflammation. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=14675 |
Introduction
One of the serious burden of society is stress which may be psychological, physiological or environmental stressors resulting in neuropsychological dysfunction like Major depressive disorder (MDD) with cognitive impairment.1,2 According to world health organization, 350 million people worldwide suffer with MDD. Repeated stressful events changes mood and cognitive decline altered by neurochemicals, of immune and stress hormones system.3 Evidences of chronic stress for functional and structural impairment in vital areas of brain such as prefrontal cortex PFC and hippocampus have increased abundantly.4,5 The PFC plays a major role in behavior, learning and memory, and also particularly hypersensitive to chronic stress.5 Preclinical and clinical studies on depression highlighted an increased cytokines expression in PFC.6,7 In addition, dysfunction in PFC’s monoamino neurotransmitters has been implicated by cytokines in the development of depression.8-10
Interestingly, these pathways implicate the path-physiology of chronic stress induced depression as explained below. Stress activates immune system and microglia cells causing production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 in PFC11 and also releases the Glucocorticoids (GC’s) via hypothalamus-pituitary adrenal (HPA) system and this is disturbed by chronic stress elevated GC’S which may increase the production of pro-inflammatory cytokines which varies in vital regions of brain.12-15Many researchers concluded that cytokines imbalance the kynurenine (KYN) pathway by the activation of indolamine-2, 3-Dioxgenase IDO, lead tryptophan depletion and reduction serotonin synaptic availability and also increases the activity of serotonin reuptake transporters in depressive condition.16,17 In animal study chronic stress model expressed cytokines and elevated IDO activity in PFC18 and another study apoptosis was detected in mice hippocampus with depression and cognitive dysfunction induced by CRS model.19,20 These indications are an important pathogenesis of emotion, depression and associated with cognitive dysfunction in chronic stress. At present most common selective serotonin reuptake inhibitors and noradrenaline reuptake inhibitors as anti-depressants are enable to suppress the expression of cytokines. According to this hypothesis we urgently need novel compound with anti-inflammatory and neuro-modulatory activities for chronic stress induced MDD.
Green Tea is freely available in nature and one of the most consumed beverages through worldwide. L-theanine is a major amino acid component (Gamma-glutamythylamide) in green tea21 and it has been shown to exhibit neuroprotective effect in various animal experimental models. Examples: antidepressant activity,22 prevent memory impairment induced by repeated cerebral ischemia in rodents.23 and anti inflammatory effect in peripheral system.24 Even it has been proven neuroprotective effect of L-theanine (2 and 4mg/kg) doses on CRS induced cognitive dysfunction with anti oxidant and anti stress activities.25
Based on recent reports we discussed above possible mechanism of stress induced neuroinflammation changes in behavior and memory impairment. In present study, we investigated the effect and underlying mechanism of L-theanine (4mg/kg) 25 on anxiety, depression and cognitive impairment induced by neuroinflammation in CRS model. In addition to that we quantified cytokines (TNF-α & IL-6) NE and 5-HT levels in PFC and morphology of hippocampus was observed. This work also suggests novel opportunities for preventing and treating depression by reducing neuroinflammation.
Materials and Methods
Animals
Adult C57BL/J male mice were housed in group on semi soft bedding with pellet, water and ad libitum. The animals are maintained under standard laboratory conditions at 25±1ºc temperature, relative humidity 50±10% and light dark cycle of 12:12h. All the animals allowed habituating to the housing facilities for at least one week prior to initiation of the procedure. The study was approved by the Institutional Animal Ethics Committee, Sri Ramachandra University (IAEC No: IAEC/XLVII/SRU/497/2016).
Restrain Protocol
In CRS procedure, mice were restrained in 50 ml (21⁄2 cm x 8 cm) falcon tube inside well-ventilated and 6h/day for three weeks (21 days) from age of 8 or 9 weeks. The mice were fitted tightly into restrainers and there is no possible to move or turn around.
Animal Groups
Each group contains 8 animals.
Group1. (Control) The mice were treated with vehicle (0.9%normal saline) from day 1 to day 21.
Group2. (CRS) The mice were treated with restrain stress + vehicle was administrated 1 hour before restrain stress from day 1 to day 21.
Group3. (L-theanine) The mice was treated with restrain stress + L-theanine was administrated 1 hour before restrain stress (4mg/kg/body weight, oral) from day 1 to day 21.
Chemicals
L-theanine (SRL chemicals India) was obtained from The I.L.E.Co, Chennai India. Neurotransmitter standards (HPLC sigma, st Louis, Mo. U.S.A) were obtained from bicorporals, Chennai, India. Primary and secondary antibodies (abcam) were obtained from Allied scientific products, Kolkata.
General Condition
After the stress cessation all subjects were underwent behavioral test (from 22nd day to 25th). Each behavioral test was conducted by the same person between 9AM to 4PM and which was recorded by camera. All mice were allowed to experimental room for adaption, 1 hour before testing. Behavior test was studied every day using one test as shown below,
| 22nd day
Open Field Test (OFT) |
23rd day
Force Swim Test (FST) |
24th day
Novel Object Recognition test (NORT) |
25th day
Y Maze Task (Y maze) |
Behavioral Test
OFT
To measure the locomotor activity and anxiety like behavior of mice we used open field apparatus.26 The apparatus are wood in material and comprised of 15.74 x 15.74 inches square arena with 7.87 inches high wall. It was divided into 25 equal squares which was marked in floor of arena. Single mice gently placed into corner of floor in order to explore the arena for five minutes. The activity of mice was recorded by a camera; number of squares was crossed by each mice was scored. In addition to that time spent in central and peripheral squares, immobility period, rearing behavior and grooming activity were scored and analyzed. After finishing the test for each mouse, floor was cleaned with alcohol to exclude the intervention of odor signal.
FST
The test was performed as described previously with minor modifications.18 To measure the depressive like behavior, mice were placed into a glass tank (diameter 23 cm & high 30cm) up to 20 cm high filled with 25 ± 1ºc water so that mice cannot reaches the bottom with their tail. Behavior (struggling: the mouse was vertical in the water, scratching vigorously the walls of the beaker; floating: no movements of the mouse except for minor movements to keep balance; swimming: all other behavior i.e. activity movement) was recorded by a camera for 5 minutes. Thereafter mice was dried with towels and shifted into a fresh cage. Water was replaced after completing the procedure for each animal.
Nort
Mice were assessed using novel object recognition task similar to that described previously.26 During habituation the mice were allowed to explore the empty apparatus for 15 minutes. During the acquisition trail (T1), apparatus contained two identical objects. A mice was taken from its home cage and placed into the apparatus, central from 2 objects facing the wall in front of the observer and time spent actively exploring (exploration was defined as the animal sniffing or touching) the objects during 5 minutes after T1, the mice was transferred into home cage. After 24 hours, the mice were again placed into the apparatus for the discrimination trail (T2). The time spent exploring the two objects during T1 and T2 was recorded and discrimination index (DI) was calculated based on the following formula:
DI = RI / (Time spent in exploring novel object + Time spent in exploring familiar object)
Recognition Index (RI) = Time spent in exploring novel object – Time spent in exploring familiar object.
Y maze
The Y maze test is less stressful and ethological more relevant to spatial memory test. Mice was assessed spatial memory function, as described previously with minor modifications.27 The maze consist of 3 arms appear as Y shape. The 3 arms of the maze randomly assigned as open arm (which is left open during training and test duration) start arm (where mice was left first during training and test duration) novel arm (which is blocked during first training period). Ocular clues were hung on the maze during the trails and test. Mice were pre-trained keeping novel arm closed. After 1 hour break test was carried keeping the novel arm is open. Novel versus familiarity of the mice was assessed by comparing the behavior in all the 3 arms. Mice were placed in start arm during all assessment. Number of visits and time spent in each arm was recorded by camera for 5 minutes.
Sample Storage
After behavioral study (i.e. 26th day), the mice was anesthetized and scarified using isoflurane, blood and brain was collected. Blood centrifuged at 3000 rpm for 10 minutes at 4ºC, plasma and brain samples were frozen at -80ºc until the analysis.
Detection of PFC Tissue TNF-Α and IL-6 by Immunohistochemistry
Paraffin section was pretreated by using heat mediated antigen retrieval with sodium citrate buffer (pH 6) for ½ hour. The sections were stained with one of the primary antibodies: Rabbit polyclonal to mouse IL-6 (ab6672, 1/200 dilution) or Rabbit polyclonal to mouse TNF alpha (ab6671, 1/200 dilution). The slides were washed with appropriate buffer and incubated with HRP conjugated secondary (ab 97023, 1/1000 dilution) for 1 hour at room temperature. Sections were washed and stained with DAP for 15mins. Counterstaining with hematoxylin was performed the slides were mounted with aqueous mounting media and visualized under light microscope. The percentage of positive cells in 5 random microscopic fields was measured by 2 independent pathologists who were blind to clinical data.
Neurotransmitters Estimation by LCMS
PFC tissues were homogenized with ice cold methanol (1mg/10µl). After homogenization samples were centrifuged at 10,000 rpm for 10 minutes at 4ºc.
LCMS Analytical Conditions
Instrument shimadzu LCMS 8040 triple quadrupole system coupled with UHPLC (NEXERA), with Inert ODS column (150×4.6mm, 5Â). Mobile phase A 0.01% acetic acid in water, Mobile phase B methanol was used. Injection volume is 10 µl, Gradient (Time/B. Conc.) 0.01/15, 5/85, 10/85, 11/15 & 15/15. Flow rate 0.7ml/min.
Histopathology Analysis
Hippocampus collected from the mice and fixed in 10% neutral buffered formalin. Representative tissue section of brain at the level of hippocampus region was trimmed and dehydrated in series of graded alcohol and embedded in paraffin wax. Tissue sections of 3-4 micron thickness was obtained, mounted on a clean glass slides and stained with haematoxylin and eosin (H&E). Morphology of hippocampus was observed by a light microscope. Affected neuron cells were identified by their pyknotic nuclei and acidophilic (esinophilic) cytoplasm, which are the suggestive of necrotic morphology.20 Semi quantitative scale used for affected cells counting (0- absent, 1-weak, 2-moderate and 3-high) in the hippocampus neuronal cells.
Corticosterone Analysis
Plasma CORT levels were estimated using the fluorometric assay of matingly (1962),28 a corticosterone stock (sigma) solution 100mg/dl was prepared and diluted to a range of concentrations 10-100µg/dl. Plasma samples and corticosterone standards were mixed in 7.5mldichloromethane. Five milliliters of the dichloromethane extract phase was transferred and mixed with 2.5ml of fluorescent reagent congaing concentrated sulphuric acid: absolute ethanol at 70:30 ratio and the tubes were thoroughly mixed. An aliquot of the lower phase was removed and measured with fluorescence at excitation 470 nm & emission 530 nm using spectrophotofluorimeter. The results were expressed as µg/dl of plasma.
Statistical Analysis
Data expressed as mean ± standard error of mean (SEM). The difference between time spent exploring the novel object versus familiar object during discrimination trail was calculated for each group and the level of significance was analyzed using two-side student’s t test. For other parameters the difference between the groups were determined using one way ANOVA followed by Tukey’s – post hoc test for multiple comparisons using SPSS software version 15.0 (cary NC). P values <0.05 were considered as significant.
Results
Behavior Assessment
OFT
CRS induced mice were made significantly (p<0.001) decrease total number of squares crossed compared to control group (Figure: 1A). There was also significant increases time spent in peripheral squares and decrease in central squares (p<0.001) in CRS group compared to control group (Figure: 1B). Treatment of L-theanine increase the number of squares crossed, time spent in central squares and decrease the peripheral squares compared to CRS group. Administration of L-theanine treated mice significantly decreased immobility period and reversed effect of rearing and grooming compared to CRS group (p<0.001).
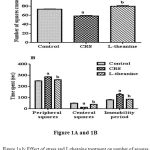 |
Figure 1a.b: Effect of stress and L-theanine treatment on number of squares crossed (Figure: 1A) time spent in central, peripheral squares and immobility period (Figure: 1B) in the open field exploration test.
|
Data expressed as mean ± SEM (n=8 per group). aP<0.001, as compared to the control group; bP<0.001, as compared to the CRS group.
FST
In FST significantly increase the floating period and decrease the swimming time in CRS compared to controlled group (p<0.001). It has shown in figure: 2 the treated with L-theanine mice significantly (p<0.001) increase the swimming time and decrease the floating period compared to CRS group.
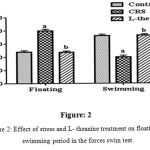 |
Figure 2: Effect of stress and L- theanine treatment on floating and swimming period in the forces swim test.
|
Data expressed as mean±SEM (n=8 per group). aP<0.001, as compared to the control group; bP<0.001, as compared to the CRS group (Figure: 2).
Nort
In NOR test CRS mice (group-2) significantly decrease number of visits and exploration time of the novel object compared to control group. Treatment of L-theanine significantly (p<0.001) increased in time spent and number of visits exploring the novel object compared to CRS group (Figure: 3A, 3B& 3C).
 |
Figure 3a.b.c: Effect of stress and L-theanine treatment on NOR test and the data expressed as mean ± SEM (n=8 per group).
|
Figure: 3A Novel object and familiar object exploration time aP<0.001, as compared to the control group; bP<0.001, as compared to the CRS group. Figure: 3B Number of novel object visits aP<0.001, as compared to the control group; bP<0.001, as compared to the CRS group and number of familiar object visits aP<0.001, as compared to the control group; bP<0.001, as compared to the CRS group. Figure: 3C discrimination of index in objective recognition test.
Y Maze
In Y maze test group-2 significantly decreased the novel arm entries and increased the open arm entries compared to control group (Figure: 4A). Treatment of L-theanine was increased novel arm entries and decreased open arm entries compared to group-2. Time spent in open arm significantly (p<0.001) increased in CRS group compared to control group, but there is no significance between L-theanine treated group and CRS group (Figure: 4B).
 |
Figure 4a.b: Effect of stress and L-theanine treatment in the Y maze task and the data expressed as mean ± SEM (n=8 per group).
|
Figure: 4A Number of entries in novel arm aP<0.001, as compared to the control group; bP<0.001, as compared to the CRS group and number of open arm entries ap<0.001, as compared to the control group; bP<0.01, as compared to the CRS group. Figure: 4B Time spent in open arm, as aP<0.001 compared to control group; bP >0.05 as compared to the CRS group.
Immunohistochemistry Analysis
Immunohistochemical analysis showed that inflammation in CRS mice PFC exhibited an increased expression of TNF-α and IL-6 when compared to the control group. However the protein expression of TNF-α and IL-6 in PFC were considerably decreased in the L-theanine treated group (Figure: 5A, 5B & 6).
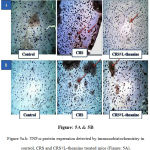 |
Figure 5a.b: TNF-α protein expression detected by immunohistochemistry in control, CRS and CRS+L-theanine treated mice (Figure: 5A).
|
IL-6 protein expression detected by immunohistochemistry in control, CRS and CRS+L-theanine treated mice (Figure: 5B). Magnification of IHC images is 40x as indicated and the scale bars = 40µm (n=8 per group).
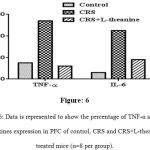 |
Figure 6: Data is represented to show the percentage of TNF-α and IL-6 cytokines expression in PFC of control, CRS and CRS+L-theanine treated mice (n=8 per group).
|
Effect of L-Theanine on Neurotransmitters Decreased in PFC
In CRS significantly (p<0.001) reduced levels of NA and 5-HT as compared to the control group (Figure: 7). However, treatment of L-theanine resulted significantly (p<0.001) increased NA and 5-HT levels in PFC as compared to the CRS group.
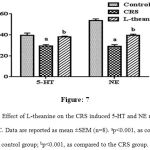 |
Figure 7: Effect of L-theanine on the CRS induced 5-HT and NE reduction in the PFC. Data are reported as mean ±SEM (n=8). ap<0.001, as compared to control group; bp<0.001, as compared to the CRS group.
|
Effect of Corticosterone Elevated Levels in Plasma
The CORT levels in plasma were significantly (p<0.001) higher in CRS group than control group (Figure: 8), while significantly (p<0.001) declined in the plasma CORT levels was observed in L-theanine treated group.
Histopathological Examination of Hippocampus
L-theanine reversed the CRS induced neuronal apoptosis in the hippocampus. The neuronal cells of hippocampus histopathological changes were observed by hematoxylin and eosin (H&E) staining. In control group, there were absent of neuronal abnormalities. However, in CRS degenerated neurons (esinophilic pyknotic neuron) were highly observed. L-theanine treated mice neuronal abnormality was absent and potentially reversed the neuronal degeneration (Figure: 9).
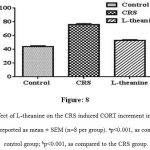 |
Figure 8: Effect of L-theanine on the CRS induced CORT increment in the plasma. Data are reported as mean ± SEM (n=8 per group). ap<0.001, as compared to control group; bp<0.001, as compared to the CRS group.
|
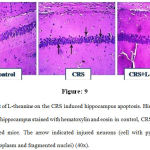 |
Figure 9: Effect of L-theanine on the CRS induced hippocampus apoptosis. Histopathological examination of hippocampus stained with hematoxylin and eosin in control, CRS and CRS+ L-theanine treated mice. The arrow indicated injured neurons (cell with pyknotic nuclei, acidophilic cytoplasm and fragmented nuclei) (40x).
|
Discussion
Based on the evidences explained in review literature of previous clinical and pre-clinical studies, the present study confirmed and expanded the information on chronic stress which produced changes in behavior and cognition status of C57BL6/J mice. Stress was induced by restrainer for 21 days (6hrs/day) and from 22nd to 25th day the animals were observed for behavioral changes and memory impairment. CRS group mice showed decrease in locomotor activity (decreased number of squares crossed), increased anxiety (decreased time spent center squares in OFT) and more depressive like behavior (increased floating time in FST). Impairment in recognition (NOR test on novel object exploration was less when compared to neither control group) and in spatial memory impairment (novel arm entries were decreased in Y maze)26,27 These behavioral and cognitive changes were in parallel with neurotransmitters such as NE and 5-HT, pro-inflammatory cytokines such as TNF-α and IL-6 were evoked in PFC,26,30 stress hormones (CORT) levels were elevated in plasma and apoptosis was found in hippocampus.20 Most abnormalities resulting from chronic restrain stress were reduced by oral administration of L-theanine. These findings support the present hypothesis that treatment with L -theanine on group 3 can counteract chronic stress induced depression, impairment in cognitive function and alterations in neurotransmitters by reducing cytokines expression.
Despite facts well known that neuroinflammation is a key factor in development of neuropsychiatric dysfunction,31,32 there is increase in TNF-α and IL-6 protein expression after CRS.33 In this present experiment, L-theanine decreased the expression of TNF-α and IL-6 in PFC shown in 5 & 6, as reported already that anti inflammatory activity of L-theanine.34 Also GCs have potential anti-inflammatory activity, but previous findings by Shawn F Sorrells et al., 2009 suggested that chronic stress which increase GCs levels, can enhance cytokines transcription factor which varies in vital regions of brain.12,15 Even in present study L-theanine decreased CORT levels in plasma (anti inflammatory activity has proven in earlier preclinical studies).25 This activity may be potentiating the anti inflammatory activity in present study.
In brain neurotransmitters were easily affected by chronic stress. NE and 5-HT levels in PFC were decreased which might be the reason for behavioral changes caused by chronic stress induced neuroinflammation.18,26 The present study L-theanine group significantly increased levels of 5-HT and NE in PFC comparatively to CRS group. Xia Tian et al., has suggested that L- theanine restored NE levels in brain which was altered by CRS.25 Reduction of serotonin levels is a major role in etiology of MDD. Increasing serotonin levels at neuroeffector junction is the most common treatment which is currently advised in depression. The present study shows, restoration of 5-HT levels in PFC of L-theanine treated group, probably following mechanism may be involved for the restoring serotonin levels in PFC. 5-HT directly correlates with its precursor of tryptophan in chronic stress evokes cytokines which elevates the IDO levels, increase catabolism of tryptophan by involving in KYN pathway and also over activates the serotonin reuptake receptors.17,18,30 The present study L-theanine inhibited the expression of cytokines which may lead to increase bioavailability of 5-HT. Previous studies concluded that L-theanine has neuro protective activity by interacting with monoamino neurotransmitters.25 S.linga et al. stated that CRS induced inflammation reduced the levels of 5-HT in PFC and recognition memory impairment determined by the object recognition test.26 In our present study, L-theanine restoration of 5-HT levels improved recognition performance in NOR test (Figure: 3A, 3B& 3C) and decreased the duration of immobility period in FST (Figure: 2). Previously Wakabayashi reported that L-theanine has antidepressant activity by increasing mobility period in FST.22 In present study treatment of L- theanine increased locomotor activity and time spent in central squares and decreased time spent in peripheral squares during OFT (Figure: 1A&B) indicating the anxiolytic and anti depressant activity.34 The hippocampus is an important region for learning and memory. In previous study spatial memory impairment in CRS model with neuronal apoptosis in hippocampus CA1 region was correlated with the activation of Akt/GSK-3 signaling pathways.19,20 In present study treatment of L-theanine prevented the spatial memory impairment shown in figure: 4A & 4b by alleviating CRS-induced neuronal apoptosis of hippocampus as shown in 9. In previous study L-theanine shown ameliorative role by inhibiting phosphorylations of GSK-3 in neuronal cells.36 Our present study result showed the effect of L-theanine as novel therapeutic compound in various long-term stress induced depression and cognitive impairment conditions. As well as these findings strongly support the possible mechanism behind the adjuvant action and present hypothesis.37
Conclusion
The group of mice kept under CRS showed features of behavioral abnormalities like anxiety, mental depression and impairment in cognition, also lowered neurotransmitter levels and expression of cytokines like TNF-α and IL- 6 in PFC resulting elevated apoptosis in hippocampus. These effects are found to be reversed in the group of mice treated with L-theanine in addition to CRS mice. This may explain the possible mechanism of preventing the cytokines involvement in PFC neurotransmitters and apoptosis in hippocampus as well. Our findings strongly suggest that L- theanine is a potential compound to prevent anxiety, depression associated with cognitive impairment, protecting PFC and hippocampus from chronic stress.
Acknowledgements
Authors would like to express my sincere thanks to Dr Rajendra Tare and Dr Yogeshkumar Murkunde (Veterinarian Pathologists) and Ramakrishna, CEFT and Lakshmi Sundaram, CRF, Sri Ramachandra University, for helping in research work.
Conflict of Interest
There is no Conflict of Interest
References
- Schneiderman N.,Ironson G., Scott D. Siegel. Stress and Health: Psychological, Behavioral and Biological Determinants. Annu Rev Clin Psychol. 2005;1:607–628.
doi:10.1146/annurev.clinpsy.1.102803.144141.
CrossRef - McEwen S. B and Peter J. Gianaros Central role of the brain in stress and adaptation: Links to socioeconomic status, health and disease Ann N Y. Acad Sci. 2010;1186:190-222.
CrossRef - Miller H. A and Raison L. C. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology. 2015;16:23-34.
- Lucassen J. P.,Pruessner J.,Sousa N.,Almeida F. X. O.,Van Dam M. A., Rajkowska G.,Swaab F. D andCzéh B. Neuropathology of stress Acta Neuropathol. 2014; 127(1): 109–135.
- Amy F. T. Arnsten Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422.
CrossRef - Pandey N. G., Rizavi S. H.,Ren X., Fareed J.,Hoppensteadt A. D., Roberts C. R., Conley R. R and Dwivedi Y et al. Proinflammatory Cytokines in the Prefrontal Cortex of Teenage Suicide Victims. J Psychiatr Res. 2012;46(1):57–63.
CrossRef - Liu W.,Sheng H.,Xu Y., Liu Y.,Lu J and Ni X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behavioural Brain Research. 2013;242(1):110–116.
CrossRef - Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34(1):4–20.
- Lichtblau N., Schmidt M. F.,Schumann R.,Kirkby C. K and Himmeri H. Cytokines as biomarkers in depressive disorder: Current standing and prospects. International Review of Psychiatry. 2013;25(5).
- Clark M. S., Pocivavsek A.,Nicholson D. J.,Notarangelo M. F.,Langenberg P.,McMahon P. R.,KleinmanE. J., Hyde M. T., Stiller J.,Postolache T. T.,Schwarcz R and Tonelli H. L. Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J Psychiatry Neurosci. 2016;41(6):386–394.
- Bollinger L. J.,Burns B. M. C and Cara L. Wellman. Differential Effects of Stress on Microglial Cell Activation in Male and Female Medial Prefrontal Cortex.Brain Behav Immun. 2016 Feb; 52: 88–97.
CrossRef - Shawn F. Sorrells, Javier R. Caso, Carolina D. Munhoz, and Robert M. Sapolsky. The Stressed CNS: When Glucocorticoids Aggravate Inflammation. Neuron 64, October 15, 2009 ª 2009 Elsevier Inc.
- Erica de Almeida Duque and Carolina Demarchi Munhoz. The proinflmmatory Effects of glucocrticoids in the brain. Front Endocrinol (Lausanne). 2016; 7: 78.
- Patricia A. Zunszain,Christoph Anacker,Annamaria Cattaneo,Livia A. Carvalho, and Carmine M. Pariante. Glucocorticoids, cytokines and brain abnormalities in depression.Prog Neuropsychopharmacol Biol Psychiatry. 2011 April 29; 35(3): 722–729.
- Pace, T. W., Hu, F. & Miller, A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 21, 9–19 (2007).
CrossRef - Andrew H. Miller, Vladimir Maletic, and Charles L. Raison.Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression.Biol Psychiatry. 2009 May 1; 65(9): 732–741.
CrossRef - Maes, M., Leonard, B. E., Myint, A. M., Kubera, M. & Verkerk, R. The new ‘5‐HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3‐dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35,702–721 (2011).
CrossRef - Weina Liua,c, Hui Shenga, Yongjun Xua, Yu Liub, Jianqiang Lub, Xin Nia. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: Relevant to proinflammatory cytokines and IDO activation. Behavioural Brain Research 242 (2013) 110–116.
CrossRef - Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, et al. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets 2006; 5:531–46.
CrossRef - Pengcheng Huang, Cai Li, Tianli Fu, Dan Zhao, Zhen Yi, Qing Lu, Lianjun Guo, Xulin Xu. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behavioural Brain Research 288 (2015) 1–10.
CrossRef - Terashima, T., Takido, J., Yokogoshi, H., 1999. Time-dependent changes of amino acids in the serum, liver, brain and urine of rats administered with theanine. Bioscience, Biotechnology, and Biochemistry 63, 615–618.
CrossRef - Wakabayashi, C., Numakawa, T., Ninomiya, M. et al. Behavioral and molecular evidence for psychotropic effects in L-theanine Psychopharmacology (2012) 219: 1099.
- Yamada, T., Terashima, T., Honma, H., Nagata, S., Okubo, T., Juneja, L.R., Yokogoshi, H., 2008. Effects of theanine, a unique amino acid in tea leaves, on memory in a rat behavioral test.Biosci. Biotechnol. Biochem. 72, 1356–1359.
CrossRef - Chengjian Li., Haiou Tong., Qiongxian Yan.,Shaoxun Tang., Xuefeng Han., Wenjun Xiao., Zhiliang Tan et al. L-Theanine Improves Immunity by Altering TH2/TH1 Cytokine Balance, Brain Neurotransmitters, and Expression of Phospholipase C in Rat Hearts.© Med Sci Monit, 2016; 22: 662-669.
CrossRef - Xia Tiana,Lingyan Suna, Lingshan Goua, Xin Linga, Yan Fenga, Ling Wanga, Xiaoxing Yina, Yi Liua et al. Protective effect of L-theanine on chronic restraint stress-induced cognitive impairments in mice. Brain research 1503 (2013) 24–32.
CrossRef - Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y andJin F et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015 Dec 3; 310:561-77.
CrossRef - Akwa Y, Ladurelle N, Covey DF, Baulieu EE. 2001. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? [J]. Proceedings of the National Academy of Sciences of the United States of America, 98: 14033-14037.
CrossRef - D Mattingly, H Martin, Christine Tylerj. Fluorimetric method for simultaneous estimation of cortisol, corticosterone, and testosterone in plasma. Clin Pathol 1989;42:661-666.
- Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P and Lima MMS et al. Chronic omega-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress; protocol. Behav Brain Res 219(1):116–122. http://dx.doi.org/10.1016/j.bbr.2010.12. 028.
- Yvonne Couch, Daniel C. Anthony, Oleg Dolgov , Alexander Revischin, Barry Festoff, Ana Isabel Santos , Harry W. Steinbusch and Tatyana Strekalova et al.Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain, Behavior, and Immunity 29 (2013) 136–146.
- Min Ma, Qian Ren, Ji-chun Zhang, Kenji Hashimoto. Effects of Brilliant Blue G on Serum Tumor Necrosis Factor-α Levels and Depression-like Behavior in Mice after Lipopolysaccharide Administration. Clinical Psychopharmacology and Neuroscience 2014;12(1):31-36. http://dx.doi.org/10.9758/cpn.2014.12.1.31.
CrossRef - Seden Demirci1, Ayşe Aynalı2, Kadir Demirci3, Serpil Demirci1, Buket Cicioğlu Arıdoğan2, The Serum Levels of Resistin and Its Relationship with Other Proinflammatory Cytokines in Patients with Alzheimer’s Disease. Clinical Psychopharmacology and Neuroscience 2017;15(1):59-63. https://doi.org/10.9758/cpn.2017.15.1.59.
CrossRef - José L.M. Madrigal, Olivia Hurtado, María A. Moro, Ignacio Lizasoain, Pedro Lorenzo, Antonio Castrillo, Lisardo Boscá, and Juan C. Leza. The Increase in TNF-Levels Is Implicated in NF-B Activation and Inducible Nitric Oxide Synthase Expression in Brain Cortex after Immobilization Stress. Neuropsychopharmacology 2002–VOL. 26, NO. 2
- Chengjian Li, Haiou Tong, Qiongxian Yan, Shaoxun Tang, Xuefeng Han, Wenjun Xiao, Zhiliang Tan. L-Theanine Improves Immunity by Altering TH2/TH1 Cytokine Balance, Brain Neurotransmitters, and Expression ofPhospholipase C in Rat Hearts. © Med Sci Monit, 2016; 22: 662-669.
CrossRef - Sumathi T.Christinal J. L-Theanine, an aminoacid in tea augments Methylmerucry (Global pollutant) induced Biochemical, Behavioural and Histological changes in rat cerebellum. International Journal of Pharmacognosy and Phytochemical Research 2015; 7(2); 286-293
- Peiling Ben, Zhengping Zhang, Yanyan Zhu, Aiying Xiong, Yanhong Gao, Jianyun Mu, Zhimin Yin and Lan Luo et al. L-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation.NeuroToxicology. Volume 57, December 2016, Pages 95–103.
- Boddu V S Chandrasekhar, Elango.P, D Rajukumar, S Umamaheswari. A pharmacological approach towards mechanisms of chronic stress induced neuroinflammation, depression and cognitive impairment. Int J Pharm Bio Sci 2017 Jan ; 8(1): P 17- 25.








