Olga Zorikova1.2, Artem Manyakhin1.2.3, Vladimir Koldaev1, Larisa Moiseenko1.2 and Ekaterina Litvinova3
1Mountain-Taiga Station behalf of Vladimir Komarov - branch of the Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch of the Russian Academy of Sciences Vladivostok, Russia.
2Vladivostok State University of Economic and Service, Vladivostok, Russia.
3Far Eastern Federal University Vladivostok, Russia.
Correspondence Author E-mail: mau84@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/1152
Abstract
The focus of the research is allelopathic activity of Patrinia scabiosifolia and Patrinia rupestris. Secondary metabolites of higher plants, which regulate growth, are of interest as the basis for possible agents to be used in crop production. In order to study the phytogenic field, soil samples were selected. Gramineous and forb plant association soil was used as control. Cucumber seeds (Cucumis sativus) were used as test culture to identify the allelopathic activity of the phytopathogenic field. The results were analyzed by methods of mathematical statistics using Statistica 6.0. The bioassays of soil samples showed pronounced phyto-activity of the samples tested, which owes to secretions of parts of the donor plants. The results obtained may be used for commercialization of P. scabiosifolia and P. rupestris material as a source of biologically active substances possessing sedative and adaptogenic properties, as well as in crop production. Metabolites of P. rupestris manifest an inhibitory action, with maximum impact on the test crop caused by generative parts and leaves. Metabolites of parts of P. scabiosifolia did not have a meaningful impact on germination of test cultures and had a stimulating effect on the development of seedlings.
Keywords
Allelopathic Activity; P. scabiosifolia; P.Phytopathogenic Field rupestris;
Download this article as:| Copy the following to cite this article: Zorikova O, Manyakhin A, Koldaev V, Moiseenko L, Litvinova E. Allelopathic Activity of Patrinia Scabiosifolia and Patrinia Rupestris. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Zorikova O, Manyakhin A, Koldaev V, Moiseenko L, Litvinova E. Allelopathic Activity of Patrinia Scabiosifolia and Patrinia Rupestris. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=14559 |
Introduction
One of the goals of Botanical Resources Studies is to discover biologically active substances and find out their physiological action so to apply it in various fields of economy. Secondary metabolites of higher plants, which regulate growth, are of interest as the basis for possible agents to be used in crop production. These substances, which cause different growth reactions depending on concentration, are generally well soluble in water and are of easy biological access.
Among cholines produced by higher plants in temperate climates such compounds are to be named as polyphenols, polysaccharides, phenol-carbonic acids, aldehydes, alcohols, ketones, essential oils, terpenes, proteins and other substances.1-2
Theoretical Background
The object of the present study is species of Patrinia genus: P. scabiosifolia and P. rupestris (Caprifoliaceae family), aborigines of the South of the Russian Far East. The presence of polyphenolic compounds in water and ethanol extracts of leaves, inflorescences, and shoots of various species of the specified genus has been recorded by researchers from Russia, Japan, China, and Kazakhstan.3-5 Secondary metabolites are known to be of high importance in interspecific and interpopulation interactions, which contribute to regulation of the species composition and the structure of a phytocenosis.6
Studies carried out in the recent decades have confirmed the widespread nature of allelopathic interaction among plants. At the same time, it has been detected that the manifestations of allelopathic effect depend on a most various range of biotic and abiotic factors. When substances of allelopathic nature secreted by plants are dissolved in soil cover or in air, they can travel long distances and have a significant impact not only on the neighboring, but also on relatively far-dwelling organisms. Around each plant within the phytogenic field, an “allelopathic sphere” is formed, which owes to plant secretions buildup in the environment.7 Therefore, the challenge happens to be to identify the allelopathic activity, which is defined as comparative ability of a plant to accumulate physiologically active substances (cholines) in the environment. These substances can either be direct metabolites of the donor plant or emerge in the course of secondary processes. Detecting either high or low activity of cholines in the allelopathic sphere of a plant we can make conclusions about its role in the phytocenosis. We have not found any information concerning studies of the impact of metabolites of the specified plants upon the formation of allelopathic mode of the phytogenic field.
The goal of this paper was to study the allelopathic activity of P. scabiosifolia and P. rupestris. One of the tasks of the research was to conduct a series of biological tests so to detect the allelopathic activity of water-soluble metabolites of various parts of the studied plants. Another task was to detect the allelopathic mode of the phytogenic field of P. scabiosifolia and P. rupestris.
Study Methods and Procedure
The territory of Primorsky Krai, where the research was carried out, is part of the natural habitat of the species studied (P. scabiosifolia and P. rupestris).
To study the phytogenic field, soil samples were selected from 20 specimens of P. rupestris and P. scabiosifolia, the ground litter from a depth of 0-5 cm being removed. The distance from the donor plant was measured as 0.5 of the projection of the part above the ground. Generative plants in the phase of fructification/early dissemination were selected as donors. Gramineous and forb plant association soil was used as control.
Seeds of cucumber (Cucumis sativus) were used as the test culture to determine the allelopathic activity of the phytogenic field. The following parameters were taken into account: length of the seed-lobe of the seedling (mm), length of the seedling stem (mm), length of the seedling root (mm). Germination energy (5 days), and germination rate (7th day) was assessed. The index of allelopathic activity was calculated with respect to the main parts of the seedling (cotyledons, stem, root).
I = (Lk-Lo)/Lk; where
I is the index of the allelopathic activity;
Lk is the morphometric index of the germ in the control;
Lo is the morphometric index of the germ in the experiment;
The activity of allelopathic substances contained in leaves, buds, stems and rhizomes of P. scabiosifolia and P. rupestris was monitored by bioassays method, i.e. by monitoring the impact of water extracts of plant material of the relevant plant parts upon the growth of sprouts of test crops: garden cress (Lepidium sativum) and radish (Raphanus sativus). Leaves, inflorescences, stems, and rhizomes were picked during the 3rd decade of August from 15 specimens of each species. General samples of parts of each species were formed from these pickings. To prepare the aqueous extract a weighed quantity of 10 g of fresh plant material was rubbed in a mortar with crushed glass. Distilled high purity H2O was added to the prepared weighed amount in 1:10 ratio, the extraction time being 40 minutes and the preparation being constantly stirred at the temperature of 40°С. Aqueous extracts were passed through an ashless filter. 150 seeds of each test culture were put for germination in plastic petri dishes, d=15 cm, on pulp pads, at a constant temperature of 23°C.
Cellulose pads were soaked with distilled water in the control dishes and with water extracts of leaves, stems, inflorescences, fruits, and rhizomes of P. scabiosifolia and P. rupestris in each test culture. Germination (%) and the length of the seedlings (mm) was recorded on the 9th day of the experiment. The experiment was carried out three times for each version (species, soil, parts). The results were analyzed by methods of mathematical statistics using Statistica 6.0.
Results
The bioassays of soil samples showed pronounced phyto-activity of the samples tested, which owes to secretions of parts of the donor plants.
The experiment showed that in the control sample (forb soil) on the 5th day of observation the germination energy of seeds of the test culture amounted to E=100%; with P. scabiosifolia soil E equaled 60%, and with P.rupestris soil it proved to be 10%.
The germination ratio on the 9th day of observations was 100, 90 and 20% respectively. By the end of the experiment, on day 21, germination of seeds of the test culture in the control soil and in P. scabiosifolia soil remained unchanged (100 and 90%), while in the P. rupestris soil it amounted to 38%. Further monitoring within 10 days did not reveal any changes of these indicators.
Physiologically active substances contained in the soil of the P. rupestris donor plant proved to cause a pronounced phytotoxic effect inhibiting germination of seeds by 62% within the area of their impact. The sample with P. scabiosifolia donor plant showed certain inhibition (10%) in comparison with the control sample.
Interesting results (Table 1) were obtained in the course of analysis of morphometric indices of seedlings of the test culture.
In the conditions of the phytogenic field of P. rupestris expressed inhibition of development of seedlings was observed. For instance, the overall length of the seedling was by 34.5% smaller than that in the control. The tissues of the developing stem proved to be most sensitive to the physiological activity of secondary metabolites of P. rupestris, showing inhibition of 47.4% in comparison with the control, whereas cotyledons appeared to be less sensitive to the impact of the donor plant lagging the control by 34.8% in their length. The inhibiting action of the phytogenic field of P. rupestris upon the development of the overground parts of the seedling seen as reliable, the soil sample showed an unreliable stimulating effect upon the root growth, which length exceeded the control by 4.7%.
Therefore, both the stage of seed germination and the stage of further development of seedlings reveal an inhibiting effect of P. rupestris soil, which speaks for the presence of aggressive allelopathic substances.
In the case of the phytogenic field of P. scabiosifolia moderate lagging from the control (by 10%) was observed at the germination stage.
Further observations revealed (Table 1) stimulation of all the development zones of the test seedlings. The overall length of seedlings exceeded the reference values by 39.6%; the length of the cotyledons, stem and root increased by 45.7; 34.5 and 67.1%, respectively. The root meristem cells turned to be most responsive to the stimulating effects of the soil.
Table 1: Morphometric indices of seedlings of the test culture (Cucumis sativus, mm (x ± Sx)
| Donor plant | Length of cotyledon | Length of stem | Length of root | Length of seedling |
| Forb soil (control) | 11.50 ± 0.96 | 117.33 ± 2.92 | 35.41 ± 2.28 | 155.14 ± 1.78 |
| P. rupestris | 7.50 ± 0.69 * | 61.75 ± 6.57 * | 37.08 ± 2.14 | 101.56 ± 1.15 * |
| P.scabiosifolia | 16.75 ± 0.84 * | 157.83 ± 3.54 * | 59.17 ± 3.58 * | 216.64 ± 2.73 * |
The experiment revealed the presence of pronounced allelopathic activity of the phytogenic field formed by P. rupestris and P. scabiosifolia generative plants, the impact of secondary metabolites of plants secreted into the soil being bidirectional, as evidenced by the values of the allelopathic index of the soil samples (Figure 1).
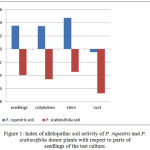 |
Figure 1: Index of allelopathic soil activity of P. rupestris and P. scabiosifolia donor plants with respect to parts of seedlings of the test culture.
|
The results of the experiment studying the allelopathic activity of extracts from parts of P. rupestris and P. scabiosifolia are shown in Table 2
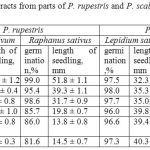 |
Table 2: Effect of water extracts from parts of P. rupestris and P. scabiosifolia on germination of seeds of test crops
|
The experiment reveals that, in the same way as in the previous experiment, P. rupestris shows a pronounced inhibitory effect. The maximum inhibition of seed germination was observed when leaves and generative parts were used, the extracts of which inhibited germination of Lepidium sativum by 73.2%, 61.5% and 75.2%. In the case of the Raphanus sativus test culture, the indicator was by 13.4%, 13.1% and 17.6% lower respectively. The lowest inhibitory effect for both test objects was observed with the extract from the P. rupestris stem.
The comparison of the seedling length indicator in each sample shows the effect of donor plant metabolites on the growth processes of the tester. The minimum growth inhibition was observed under the action of substances extracted from the rhizome of P. rupestris: the difference with the control amounted to 24.1% (Raphanus sativus) and 46.2% (Lepidium sativum). The phytotoxic effect of physiologically active substances from parts of P. rupestris upon the seedlings of test crops proved to increase as follows: rhizome<stem<leave<inflorescence<(≈) fruit. The maximum inhibition of growth processes amounted to 73.4 and 89.3%.
Test objects showed different degrees of allelopathic tolerance towards the effects of extracts. Seeds and seedlings of Raphanus sativus proved to be less susceptible to the effects of the specified extracts.
Metabolites of P. scabiosifolia showed no inhibitory effect upon germination of seeds of test crops, the indicators being close to the control in both cases. A stimulating effect of the extracts of parts of P. scabiosifolia upon the length of the seedlings of the test cultures was revealed. It was found that the underground parts and the stem of the plant investigated did not accumulate significant amounts of physiologically active substances: the increment was less than 10% in the case of Lepidium sativum and was not evident with Raphanus sativus tester. Allelopathic substances of the above ground generative parts stimulated growth by nearly 25% (22.0-24.8%) with the Lepidium sativum tester and 35% (33.9 -34.9%) with Raphanus sativus. The extract of leaves increased the length of seedlings by 23.2% and 18.8%, respectively.
Discussion
The experiment revealed an expressed allelopathic activity of the phytogenic field of natural populations of P. rupestris and P. scabiosifolia. Accumulation of cholines in dead litter in the root zone of plants has been recorded for many perennial grasses. The index of allelopathic activity (Figure 1) varies widely. The stem tissues of the seedlings turned to be most sensitive to the inhibitory action, while root tissues ended up showing the highest susceptibility to the stimulating effect.
The allelopathic activity involves a complex blend of physiologically active substances emitted by donor plants. These substances affect the growth of other plants not only by their direct toxicity, but also in an indirect way, which occurs due to the influence of biotic and abiotic factors of the soil upon metabolites.8-9 Plants create a certain mass of organic matter per area unit, thus being the original source of cholines. The plants withering away, the substances interact with biotic and abiotic soil factors, mineralizing and partially turning into permanent humus. During this conversion process, organic substances are present in various physiologically active forms, capable of providing an oligodynamic effect upon organisms. Our phyto-chemical analysis 10-11 which had been performed earlier, revealed that both species studied contain significant amounts of polyphenols, organic acids, polysaccharides, which can provide both inhibitory and stimulatory effect on seedlings.
The phytogenic field of P. rupestris provides an expressed toxic effect on the development of the overground parts of the test culture and a weak stimulating effect upon the tissues of the root, whereas the secondary substances emitted by P. scabiosifolia into the soil, significantly stimulate the development of all the parts of the germ, which is confirmed by the allelopathy index values.
One of the main groups of cholines, indicated by quite a lot of researchers, are polyphenols. Phenol derivatives, i.e. predecessors of humic acids, get into the phytocenosis environment washed out by precipitations, in the course of active secretion, or with faded parts of a plant. In the soil these substances undergo changes, which involve their amplification and polymerization of molecules and, at the same time, a simultaneous decrease in their physiological activity. The natural habitat of P. scabiosifolia are grasslands characterized by varying degrees of dryness: from overland to dampish, where the soil samples were selected from. The main ecotope of P. rupestris are rocks and rocky debris, where soils are characterized by a high degree of drainage and low humidity. As known from literary sources, under conditions of higher humidification, the process of converting cholines and the decrease of allelopathic activity of the phytogenic field is faster, while in conditions of low humidity cholines retain their high activity for a longer time.12 The qualitative and quantitative content of the major groups of metabolites being similar in plant material of the studied species, the bidirectionality of physiological activity of their cholines can be explained by the change of their concentration in the soil as a result of edaphic factors. The ability of cholines to stimulate or inhibit growth processes of the surrounding organisms by forming a phytogenic field has a significant impact on the biocenosis condition. Long-lasting joint impact of the phytogenic field formed by different species leads to the transformation of the phytocenosis.13 Thus, the allelopathic mode of the species in the study, whose physiological action is expressed by the allelopathy index, appears to be a special biogenic ecological factor.
To better understand the contribution of specific parts of the donor plants into the allelopathic sphere, an experiment was carried out focused on studying the allelopathic activity of plant parts. The most common secondary metabolites of higher plants are phenolic compounds, which usually produce a toxic effect in phytocenoses,14 which can be explained by their high biological activity and quite high resistance to the impacts of soil microorganisms. Polyphenols are allelopathic inhibitors involved in the processes of respiration and photosynthesis, as well as regulate growth and development of plants. The nature of impact of these substances upon acceptor plants has to do with the position of hydroxyl groups in the benzene ring. It is known that when affecting a plant organism in high doses, allelopathic active substances inhibit mitosis, inhibit photosynthesis, reduce the speed of movement of protoplasm, inhibit ATP-phase activity of plasmolemma, which on the organism level manifests itself as inhibition of germination and growth of seedlings in particular.15 The effect of phenolic compounds, like phytoexcrements, aims at cell membranes. These compounds affect the permeability of membranes and the activity of membrane-bound enzymes, which causes changes in energy and metabolic processes. In case of sufficiently long and intensive action, inhibition of growth and destructive changes in the acceptor plant are observed.16
The study showed that metabolites of P. rupestris manifest an inhibitory action, with maximum impact on the test culture provided by generative organs and leaves, whereas stem and underground parts exhibit a significantly smaller effect.
Metabolites of parts of P. scabiosifolia did not provide a meaningful impact on germination of the test cultures and had a stimulating effect on the seedling development, which happened to be most pronounced in cases of extracts of leaves and generative organs.
The studies of P. rupestris and P. scabiosifolia material conducted earlier showed that a larger number of substances able to impact growth processes (saponins, polyphenols, organic acids) are contained in leaves and inflorescences of plants, which coincides with the results of the present experiment.
Metabolites of P.rupestris show a selective allelopathic effect when exposed to test crops seeds: seeds of Raphanus sativus are more tolerant to the impact of allelopathic substances than seeds of Lepidium sativum (Table 2). For example, for Lepidium sativum the difference in germination rate between the control and the test sample under the impact of fruit metabolites, amounted to 75.2%. At the same time, for Raphanus sativus seeds, this difference equaled to 17.6%. This speaks for high sensitivity of Lepidium sativum to the effects of substances of P.rupestris. No such effect was observed in the case of P. scabiosifolia.
The analysis of changes of the “length of the seedling” indicator, the test culture being affected by the substances of P.rupestris, showed persistence of high sensitivity of Lepidium sativum (difference with the control — 89.3; option” fruits “) and reduced tolerance for Raphanus sativus, in which case the difference between the test and the control increased to 72%. The analysis of similar option in the case of P. scabiosifolia showed high sensitivity of the “length of the seedling” indicator for both test crops. The difference with the control was 24.8% and 34.9% for Lepidium sativum and Raphanus sativus, respectively.
It is known that small-seeded species, including Lepidium sativum, are more exposed to the impact of substances causing an allelopathic effect. Inhibition of germination of small-seeded species under the influence allelopathic substances can be explained by the larger proportion between the seed surface and its volume. As a result, the amount of allelopathic substances per mass unit is also larger compared to larger seeds. Perhaps the larger size explains the greater tolerance of seeds of Raphanus sativus. “The length of the seedling” indicator speaks for high sensitivity of both test cultures towards metabolites of both species studied.
The bidirectionality of the impact of cholines of the phytogenic field and extracts from the parts of plants in the experiment can possibly be explained by the quantitative difference of physiologically active substances of P. rupestris and P. scabiosifolia.
Conclusion
Cholines, secreted into the soil by P. rupestris, have a pronounced phytotoxic effect upon the growth processes, suppressing sprouting and development of seeds of the test-culture.
Physiologically active substances, secreted into the soil by P. scabiosifolia have an expressed stimulating effect on growth processes of the test culture seeds. rupestris and P. scabiosifolia are one of the environmental factors determining the structure of plant communities within its habitat. The buildup of allelopathic substances within the area of the donor plant affect colonization of sites by other plants, which contributes to the formation of the structure of the phytocenosis.
Compounds from leaves and generative parts of P. rupestris have a pronounced inhibitory effect on germination and development of test crops.
Compounds from leaves and generative parts of P. scabiosifolia have a pronounced stimulating effect on development of test crops.
The allelopathic activity of parts of P.rupestris and P. scabiosifolia manifests itself from minimum to maximum in the following way: rhizome <stem <leave <inflorescence <(≈) fruit.
The degree of manifestation of allelopathic activity of metabolites depends on the species specificity of test cultures.
References
- Harborne J. B., Crawley M. J. Plant secondary metabolism. Plant Ecology. Oxford: Blackwell Science. 1997;132-155. DOI: 10.1002/9781444313642.ch5.
CrossRef - Hiradate S., Morita S., Furubayashy A., Fujii Y., Harada J. Plant growth inhibition by cis-cinnamoyl glucosides and cis-cinnamic acid. Journal of Chemical Ecology. 2005;31(3):591-601. https://www.ncbi.nlm.nih.gov/pubmed/15898503.
CrossRef - Nakanishi T., Tanaka K., Murata H., Somekawa M., Inada A. Phytochemical Studies of Seeds of Medicinal-Plants. Ursolic Acid and Olganolic Acid Glycosides from Seeds of Patrinia scabiosiefolia. Fischer. Chern. Pharmac. Bul. 1993;41(1):183-186. https://www.ncbi.nlm.nih.gov/pubmed/8448818.
- Yang X. P., Li E. W., Zhang Q., Yuan C. S., Jia Z. J. Five new iridoids from Patrinia rupestris. Chem. Biodivers. 2006;3(7):762-770. DOI:10.1002/cbdv. 200690078.
CrossRef - Yang X. P., Yuan C. S., Jia Z. J. Chemical constituents from the roots of Patrinia rupestris. J. Chin. Chem. Soc. 2007;54(2):459-463. DOI:10.1002/jccs.200700064.
CrossRef - Macias F. A., Galindo J. L. S., Galindo J. C. G. Evolution and current status of ecological phytochemistry. Phytochemistry. 2007;68:2917-2936. DOI: 10.1016/j.phytochem. 2007.10.010.
CrossRef - Uranov A. A. Phitogenic field. In: Problems of Modern Botany. 1965;1:251-254. Moscow: Nauka.
- Li Z. H., Wang Q., Ruan X., Pan C. D., Jiang D. A. Phenolics and Plant Allelopathy. Molecules. 2010;15:8933-8952. DOI: 10.3390/molecules. 15128933.
CrossRef - Scognamiglio M., D’Abrosca B., Esposito A., Pacifico S., Monaco P., Antonio F. Plant growth inhibitors: all elopathic role or phytotoxic effects? Focus on Mediterranean biomes. Phytochem. Rev. 2013;12:803. DOI:10.1007/s11101-013-9281-9.
CrossRef - Yu A. M., Zorikova O. G., Nazarov D. S. Chemical composition of Patrinia rupestris. Pacific Medical Journal. 2014;2:28-29. http://elibrary.ru/item.asp?id=22002282.
- Zorikova O. G., Yakimenko L. V. Chemical analysis of Patrinia scabiosifolia. Pacific Medical Journal. 2013;2:61-63. http://elibrary.ru/item.asp?id=20191608.
- Grozdinsky A. M. Allelopathy of plants and Soil Fatigue. Kiev: Naukova dumka. 1991.
- Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. Microbial interactions in the rhizosphere: beneficial influences of plant growth promoting rhizobacteria on nutrient acquisition process. A review. Biol Fertil Soils. 2012;48:123. DOI: 10.1007/s00374-011-0653-2.
CrossRef - Andersen O. M., Markham K. R. (Eds.) Flavonoids: Chemistry, Biochemistry and Applications. CRC Press,Taylor & Francis Group. 2006;397-471. ISBN 9780849320217.
- Basotra R., Chauhan S., Todaria N. P. Allelopathic effects of medicinal plants on food crops in Garhwal, Himalaya. J. Sustainable Agr. 2005;26(3):43-56. DOI: 10.1300/J 064v26n03_06.
CrossRef - Roshchina V. D., Roshchina V. V. Secretory function of higher plants. Moscow: Nauka. 1989.








