Mohammad Ali Mapar1, Mehri Maghsoodi2, Kamal Maghsoodi3, Ali Kardooni4 and Anoosh Shafiee4
1Department of Dermatology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2Psychiatrist, Iran University of Medical Sciences, Tehran, Iran.
3Department of Cardiology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
4Department of Dermatology, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran.
Corresponding Author E-mail: mehrimaghsoodi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1084
Abstract
Warts are one of the contagious viral diseases that can cause disturbing cosmetic problems. Though there are several treatments for warts, none of them offer an effective cure with low complication for all patients. So, the aim of this study was to compare the efficacy of formalin 5% solution with placebo in treatment of plane warts. This is a controlled double-blind clinical trial study, conducted on 36 patients with plane warts referring to Imam Khomeini Hospital in Ahvaz in 2015. The patients were randomly assigned into two groups. The first group applied formalin 5% solution on their lesions, and the second group treated with normal saline as placebo twice daily for 8 weeks. Assessments for the response to treatment and side effects were performed every two weeks. Also Visual Analogue Score (VAS) at the end of treatment, and relapses were investigated after 12 weeks. The collected data was analyzed by the SPSS software. In this study, 27 women and 9 men with the average age of 17.17) ranging from 4 to 46 years) were enrolled. Formalin application was effective in 83.3% of patients, but complete disappearance of warts was seen in 11.1%. There was not any significant response to treatment in placebo group. The result of response to treatment and VAS was significantly different between two group (P>0.05). There was not any significant difference between response to treatment and age, duration of warts, warts location and number of warts (P>0.05). No noticeable adverse events were reported in the use of formalin. Also, relapse was observed in none of the patients. Results of this study demonstrated that application of 5% formalin solution is a safe and effective treatment for plane warts with few side effects and good compliance.
Keywords
Formalin; Topical; Wart
Download this article as:| Copy the following to cite this article: Mapar M. A, Maghsoodi M, Maghsoodi K, Kardooni A, Shafiee A. Comparison Between Efficacy of Formalin 5% Solution and Placebo in Treatment of Plane Warts. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Mapar M. A, Maghsoodi M, Maghsoodi K, Kardooni A, Shafiee A. Comparison Between Efficacy of Formalin 5% Solution and Placebo in Treatment of Plane Warts. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=14035 |
Introduction
Warts are one of the most common skin diseases worldwide caused by the human papillomavirus (HPV). HPV infects the squamous epithelium and can cause cell proliferation (1,2). This infection is very common in childhood, its peak incidence is at a young age, but can occur at any age. Although the exact incidence of common warts is unknown, the rate in the general population is estimated to be 7-10%, and among children 20 %( 2, 3, 4).
Warts are self-limiting, they recover without treatment within several years (5). In general, spontaneous recovery is related to the immune system and a full cellular immune defense of the epidermis is needed to clear HPV virus (1). So, although spontaneous recovery occurs, treatment is usually needed, because of the cosmetic problems, pain, concerns about the development of malignant changes, psychosocial complications, unpredictable course(3,6) and contagion(7).
There are different treatments for warts, including chemical (salicylic acid) and physical (cryotherapy) destructive methods, using virucidal and anti-proliferative agents and immunotherapy. The best treatment could differ based on the patient’s age, side effects, location of lesions, and patient’s request (8, 9, and 10). However, due to the lack of specific antiviral treatment for HPV, wart treatment remains a challenging problem (1).
There are different types of skin warts on the basis of their morphology or location, including: common warts, flat warts, filiform warts, periungual warts, and palmoplantar warts (4, 8).
Flat warts are usually more resistant to treatment than what common warts show, and since these kinds of warts often occur in areas that are important in terms of beauty, use of destructive methods does not seem desirable in these areas considering the possibility of scarring(1).
Moreover, efficacy of physical and destructive therapies has not been studied in clinical trials and has not been established. Many studies about the topical treatment of warts, especially flat type warts, are not reliable, because of poor methodology and reporting. Therefore, studying the effects of topical treatments is highly recommended (6, 11).
Formalin (formaldehyde) is a virucidal agent and has strong disinfectant properties, and exerts its effects by causing damage to the upper layers of epidermal cells that contain the virus, and thus destroying viruses (12, 13). The most common side effects of formalin include redness, irritation and dryness of skin (15). Severe allergic reactions are rare (15, 16).
Given that no single choice and effective first-line treatment of warts is available, and most treatments have been limited by complications, and have been effective in only a few patients(17,18), and also due to the prevalence of warts in society and psychosocial complications caused by them, choosing a safe and effective treatment is essential.
Since most studies have focused on the effects of current treatments on common and palmoplantar warts, this study was performed to determine the effectiveness of topical formalin as an affordable, accessible medicine with low side effects in the treatment of flat warts.
Method
This study is a pilot double blind randomized clinical trial (RCT), which was designed to determine the effect of topical 5% formalin solution for treatment of flat warts. The study population consisted of 36 patients with flat warts, who were older than 4 years and referred to the dermatology clinic of Ahvaz Imam Khomeini Hospital in 2015. An allergy to formaldehyde, weakened immune systems, severe dermatitis, diabetes, pregnancy and lactation, a history of respiratory diseases including asthma, receiving other treatment for warts simultaneously, and taking immunosuppressive drugs, were set as exclusion criteria.
Before entering the study, the patients were given enough information on the health benefits and side effects of formalin, and also methodology of the study; and informed consents were obtained.
Patients were randomly divided into two groups of treatment (topical 5% formalin solution) and placebo (normal saline as placebo). Wart treatment was performed twice a day for 8 weeks, meaning that patients applied formalin solution or placebo on the wart with a cotton swab for one minute each time.
To ensure compliance with requirements of a randomized double-blind study, medications were prescribed for patients randomly and also patients, hospital staff and researchers did not know which medication had been prescribed until the end of treatment. Before starting treatment, each patient’s demographic data (age and sex) and clinical information, including the location and number of warts were recorded for each person. Patients were followed every two weeks for eight weeks by examining the number of lesions and recording side effects, and photography (with camera canon IXUS 950, 8-megapixel) took place. The patients were evaluated at the end of week 8, and then those patients in both groups who had not responded to treatment were treated with cryotherapy.
Finally, 12 weeks after the end of treatment patients were followed up again for possible relapse, response to treatment, and recovery rate from the patients’ perspective (On the VAS scale). VAS is a self-assessment tool measuring ten score (from 0 to 10), which was used to assess patients’ satisfaction. A zero result indicates no improvement, and ten indicates complete remission from the patient’s perspective (19).
Evaluation of the response to treatment was performed based on physical examination by a dermatologist in each follow up session. So that, improvement of all lesions was considered as complete response, a 75-99% reduction in the number of lesions was considered a good response, 50-74% an intermediate response, 25-49% a low response, and a reduction by less than 25% in the number of lesions was considered as a lack of response to treatment.
To analyze the data and methods used in this study, and to investigate the relationship between the variables non parametric Mann-Whitney test and Will Kalsvn test, and Chi-square analysis methods were used. Statistical analysis was performed using SPSS version 22 and the significance level was set at 0.05 in the above tests.
Results
The mean age of the participants in the study, was 17.7 years (ranging in from 4 to 46 years), and the mean age in formalin and placebo groups, was respectively 16.44 and 17.89 years. Most of the participants (63.9%) were younger than 13 years. The average duration of warts, as reported by patients in formalin and placebo groups, was 3.61 and 4.77 month, respectively. The results of statistical analysis showed no significant difference between age, gender, location of warts, warts number and duration of warts between the two groups (P>0.05). Frequency and characteristics of each variable studied in both groups are shown in Table 1.
Table 1: Profile of patients
| Demographic and clinical characteristics | Formalin | Placebo | Total |
| Frequency (%) | Frequency (%) | Frequency (%) | |
| Sex | Male: 3 (16.7) | Male: 6 (33.3) | Male: 9 (25%) |
| Female: 15 (83.3) | Female: 12 (66.7) | Female: 27 (75%) | |
| Duration of warts | 1-2 m: 4 (22.2) | 1-2 m: 3 (16.7) | 1-2 m: 7 (19.5) |
| 3-4 m: 9 (50) | 3-4 m: 8 (44.4) | 3-4 m: 17 (47.2) | |
| 5-6 m: 5 (27.8) | 5-6 m: 4 (22.2) | 5-6 m: 9 (25) | |
| 7-12 m: 0 | 7-12 m: 3 (16.7) | 7-12 m: 3 (8.3) | |
| Warts location | Face: 12 (66.7) | Face: 13 (72.2) | Face: 25 (69.5) |
| Hand: 5 (27.8) | Hand: 4 (22.2) | Hand: 9 (25%) | |
| Foot: 1 (5.5) | Foot: 1 (5.5) | Foot: 2 (5.5) | |
| Number of warts | <3: 2 (11.1) | <3: 2 (11.1) | <3: 4 (11.1) |
| 4-10: 8 (44.4) | 4-10: 6 (33.3) | 4-10: 14 (38.9) | |
| 11-20:1 (11.1) | 11-20: 4 (22.3) | 11-20: 5 (13.9) | |
| >20: 7 (38.9) | >20: 6 (33.3) | >20:13 (36.1) |
The results of statistical analysis in formalin group shows significant difference between the number of warts at different stages of treatment (P<0.05), and only between baseline and first follow-up there was no significant difference (P=0.07). In the placebo group, no significant difference was observed in the number of warts during treatment (P>0.05). The average number of warts before treatment and at the end of treatment in formalin group was 23.83 and 14.94 respectively. The amount in the control group was 18.38 and 17.44 respectively. The trend of the number of warts is shown in Figure 1.
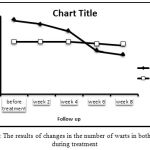 |
Figure 1: The results of changes in the number of warts in both groups during treatment
|
The results did not show any side effects in placebo group. Whereas in formalin group, in the first follow up 2 patients, in the second follow up 17 patients, and in the last two follow-ups all 18 patients showed side effects of the drug. These complications included scaling in 12 cases, fissuring and cracking of the skin in 2 cases, mild erythema in 3 cases, and severe erythema in one case, and all cases mentioned some degree of dryness and itching. Also the results of statistical analysis in the last three follow ups showed significant difference in the incidence of drug-induced complications in the two groups (P=0.000), but on the first follow up there was no statistical difference between the two groups (P=0.051).
In the formalin group, 2 patients had complete response to treatment, good response to treatment was observed in no patients, 7 patients showed intermediate response, 6 patients showed low response, and 3 patients had no response. Only one patient in the placebo group showed low response after 12 weeks (Figure 2). The results of the statistical analysis also showed significant differences between the percentage of response to treatment in both groups (P=0.000).
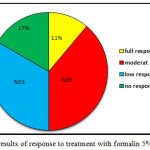 |
Figure 2: The results of response to treatment with formalin 5% of the sample
|
After 12 weeks, no relapse was observed in the course of treatment.
Scores of the VAS scale was zero at the end of their treatment in placebo group (except for 4 patients who scored 1). In the majority of patients in formalin group VAS scale score was between 3 and 6. The results of the statistical analysis showed significant differences in VAS scale score between the two groups (P=0.000).
The results of this study, didn’t show a statistically significant relationship between the rate of response to formalin and age of patients, duration of warts, the number of warts, and location of warts (P>0.05) (Table 2). However, a significant difference between sex and response to treatment was observed (P = 0.039); female gender responded better than male. There was no significant correlation between the location of warts and response to treatment. (P =0.528).
Table 2: Results of the relationship between different variables and effectiveness of formalin 5% solution in treatment of warts
| response to treatment | Mean | Max | Min | p-value | |
| Age | complete response | 7 | 7 | 7 | 0.726 |
| (year) | intermediate response | 20.57 | 4 | 44 | |
| low response | 15.33 | 4 | 45 | ||
| no response | 15.33 | 7 | 26 | ||
| Duration of warts | complete response | 1 | 1 | 1 | 0.18 |
| (month) | intermediate response | 4.14 | 2 | 6 | |
| low response | 4 | 2 | 6 | ||
| no response | 3.33 | 3 | 4 | ||
| Number of warts | complete response | 4.5 | 4 | 5 | 0.063 |
| intermediate response | 19 | 4 | 55 | ||
| low response | 25.17 | 3 | 54 | ||
| no response | 45.33 | 2 | 81 | ||
| VAS | complete response | 10 | 10 | 10 | 0 |
| intermediate response | 5.57 | 4 | 7 | ||
| low response | 3.33 | 2 | 4 | ||
| no response | 2 | 0 | 4 | ||
| * | The good response to treatment was observed in any of the subjects. | ||||
Discussion
Treatment of warts is a common clinical problem for patients and dermatologists and there are different ways to treat it. No single treatment has been reported to result complete response in all patients without recurrences, so various treatment methods have been used(20). In our study, formalin 5% solution was used, as a new drug for the treatment of flat warts.
Treatment with formalin 5% provided a statistically significant decrease in the number of warts in all stages of follow up, except for the first session; i.e. there was no significant difference between the number of warts at baseline and first follow-up; which may indicate that formalin doesn’t have a quick effect on the treatment of flat warts. Also the control group patients showed no response to treatment. So, it is not likely that flat warts, if untreated, will improve or have spontaneous recovery over a period of 3 months.
In the group treated with formalin, all patients had drug-induced side effects. In the second week, side effects include scaling, erythema, fissuring and dryness of skin. Therefore, formalin can cause dermatitis and its use should be avoided in patients with eczema (12). Patients had no signs of systemic or significant side effects in this study.
In this study, for none of the patients responding to formalin recurrence was observed. While in many treatments available for different types of warts, recurrence has been observed in most cases (21).
Although most treatment methods will lead to improved warts in 1-6 months, in 20-30% of patients relapse occurs as a result of the failure of the cellular immune system to identify and clean up the viruses (12). In the studies that was done by Talbot and colleagues (3), and McKnight and Obst (22), as in our study, recurrence was not observed in any patients treated with formalin.
In our study, response to treatment was assessed at the end of three months and in most patients (72.2%) response was moderate to low.
In the study that was done by Vickers (1961), in the patients who were younger than 17 years old, after 16 weeks of treatment, rate of improvement of plantar warts was 95.5%. In their study, formalin concentration was 3% at the start of treatment, and was further increased to 5 to 10% if no complication had happened (23). Compared to our study, the younger age of patients and use of a higher concentration of formalin might explain higher rates of improvement in their study.
McKnight and Obst reported a 90.3% cure rate over a period of 3 months of treatment with formalin 5% in patients with plantar warts, and complete cure after 6 month. Their study shows that the efficacy of a topical treatment can be equal to invasive surgery under general anesthesia (22).
In a clinical trial that was performed by Jennings et al (2006), the effectiveness of monotherapy by formalin 10% solution and combination-therapy by monochloroacetic acid (MCAA) and formalin 10% solution on plantar warts were similar, and an improvement rate of 61.4% was reported, with no significant difference in response between the two groups(24).
In another study, 200 children with plantar warts were treated with formalin 3% for 6-8 weeks. At the end of the treatment, a success rate of 80% was observed (13).
In a controlled clinical trial, no significant difference was observed between placebo and formalin 3% in 192 patients with plantar warts. Both had provided recovery rate of 61% to 67% within 2 months (25). Also, Anderson and Shirreffs (1963) didn’t show a significant difference between response to treatment with formalin 3% and placebo (26). Two studies which are antithetic with the result of our study.
In a study was performed by Talbot and colleagues in 2011, the effectiveness of formalin 10% solution was examined in 5 patients with plantar warts, and the results showed that after six months, only one case recovered and no significant difference in the recovery rate was found between case and control groups(3). Inconsistent with our results, but the sample size of the study was very small.
The results of our study didn’t show a statistically significant relationship between age, disease duration, location and number of warts, and response to treatment with formalin. Subjectively, outcomes were more favorable with shorter duration and fewer number of warts, but the data analysis did not provide support.
In our study women showed better response to treatment with formalin than men. But in the study that was performed by Anderson and Shirreffs, the response to formalin treatment was not influenced by gender or duration of warts and also patients with multiple warts responded better to treatment than those with unique wart, and response to treatment was lower with increasing age (26).
In the present study the effectiveness of formaldehyde is less than some of the other methods to treat warts. One reason for the difference in response rates, can be differences in the populations studied. In some studies, only children are the target population, while in this study, there was no age limit for inclusion. In addition to the sample size, duration of treatment can also affect the results.
Another reason for the difference in results can be attributed to the differences in the type and location of the warts. Most studies have included only common and plantar types, that show less resistance to treatment, while response of flat warts to treatment are less satisfactory, and this type of warts are more resistant to treatment than ordinary warts(1). It can be postulated that with prolonged treatment, or use of higher concentrations of formalin (up to 10%), better results could be expected.
Conclusion
Results of this study showed that administration of topical 5% formalin solution is not associated with pain or serious side effects, and can be used as an inexpensive and safe method in the treatment of flat warts, without lesions recur. But because this study is probably the first controlled clinical experience in the use of formalin in the treatment of flat warts, for definitive conclusions and decisions about treatment of warts with formalin administration, more controlled studies are needed in future.
References
- Sterling JC, Gibbs S. Haque Hussain SS, MohdMustapa MF, Handfield-Jones SE. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. BJD 2014; 171:696-712.
CrossRef - Androphy E, Lowy DR. Warts. In: Wolff K, Gold smith LA, Katz SI, Gilchrest BA, Paller AS, Lefell DJ, editors. Fitzpatrick’s Dermatology in GenaralMedicin. 8th ed. MC Graw 2012; 2119:2130.
- Talbot K, Scharfbillig R, Jones S. The effectiveness of 10% formalin in the treatment of plantar warts- A pilot RCT. UniSA. 2011; available from: http://ura.unisa.edu.au/R/?func=dbin-jump-full&object_id=60699
- Lipke MM. An armamentarium of wart treatments. Clin Med Res 2006; 4(4): 273-293.
CrossRef - Bacelier R, Johnson SM. Cutaneous warts: an evidencebased approach to therapy. Am FAM Physician 2005; 72: 647-652?
- Gibbs S, Harvey I. Topical treatments for cutaneous warts (Review). The Cochrane Library 2009; Issue1.
- Sterling JC, Han Field, Jonse S, Hudson PM. Guidelines for the management of cutaneous wart’s. Br J Dermatol 2012; 144:4-11.
CrossRef - Stulberg DL, Hutchinon AG. Molluscumcontagiosum and warts. Am Fam Phsician 2003; 15; 67(6):1233-40.
- Sterling JC. Virus Infections. In: Burns T, Breathnach S, Cox ZN, editors. Rook’s Textbook of Dermatology, 7th ed: Blackwell Publishing. 2004; 25: 37-53.
CrossRef - Gibbs S, Harvey I. Topical treatments for cutaneous warts. The Cochrane Database of Systematic Reviews 2006; 231:124-129.
CrossRef - Micali G, Dalloqlio F, Nasca MR, Teeschi A. Management of cutaneous warts: an evidence-based approach.Am j ClinDermatol 2004; 5(5):311-7.
CrossRef - Leman JA, Benton EC. Verrucas. Guidelines for management. Am J ClinDermatol2000; 1:143-149.
CrossRef - Arıcan Ö. VerrukalardaGüncelTedavi. Dermatose 2004; 3:159-159.
- Sterling JC, Handfield-Jones S, Hudson PM. British Association of Dermatologists. Guidelines for the management of cutaneous warts. Br J Dermatol 2001; 144(1):4-11.
CrossRef - Sutana R, Alam M, Khondker L, Ahmed RS. Safety in use of cryotherapy and topical salicylic acid with lactic acid combination in treating verruca vulgaris. Mymensight Med J 2012 Oct; 21(4):715-22.
- Grillo E, Boixeda P, Ballester A, Miguel-Morrondo A, Truchuelo T, Jean P. Pulsed dye laser treatment for facial flat warts. DermatolTher 2014; 27(1): 31-5.
CrossRef - Bellew SG, Quartarolo N, Janniger CK. childhood warts: an update. Cutis 2004; 73(6): 379-384.
- Han TY, Lee JH, Lee CK, Ahn JY, Soe SY, Hong CK. Long pulsed Nd: Yag laser treatment of warts report on a series of 369 cases. Journal of korean medical science 2009; 24(2): 889-93.
CrossRef - Gould D .Visual analogue scale (VAS). J Clin Nursing 2001; 10:697-706.
CrossRef - Baceliri R, Jhonson SM. Cutaneous warts: an evidence-based approach to therapy. Am Fam Phsician 2005; 15; 72(4):647-52.
- Brentjens MH, Yeung-Yue KA, Lee PC, Tyring SK. Human papillomavirus: a review. DermatolClin 2002; 20(2):315-31.
CrossRef - McKnight AG, Obst D. Assessment of treatment of plantar warts. Ulstsr Med J 1968; 37(1): 40-42.
- Vickers CFH. Treatment of plantar warts in children. Br Med J 1961; 16(2):743-745.
CrossRef - Jennings MB, Ricketti J, Guadara J, Nach W, Goodwin S. Treatment for simple plantar verrucae: monochloroacetic acid and 10% formaldehyde versus 10% formaldehyde alone. J Am Podiatr Med Assoc 2006; 96(1):53-8.
CrossRef - Gibbs S, Harvey I, Sterling JC, Stark R. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2003; (3):CD001781.
CrossRef - Anderson I, Shirreffs E. The treatment of plantar warts.British medical journal 1961; 2:743-5.
CrossRef








