Svetlana Yuryevna Zavalishina1,2, Yury Anatolevich Vatnikov3, Olga Nikolaevna Makurina4, Evgenij Vladimirovich Kulikov3, Elena Dmitrievna Sotnikova3, Valentina Ivanovna Parshina3, Ekaterina Olegovna Rystsova3, Margarita Vasil'evna Kochneva3 and Nikolaj Vladimirovich Sturov3
1Kursk Institute of Social Education, Russian State Social University, Kursk, Russia.
2All-Russian Research Institute of Physiology, Biochemistry and Nutrition of Animals, Institute of village, Borovsk, Russia.
3Peoples' Friendship University of Russia, veterinary Faculty, Moscow, Russia.
4Samara State Aerospace University named after academician S.P.Korolev (National Research University), Department of Biology, Samara, Russia.
Corresponding Author E-mail: ilmedv1@yandex.ru
DOI : https://dx.doi.org/10.13005/bpj/1090
Abstract
to appreciate physical loads’ influence on rising intravascular thrombocytes’ activity of healthy mice during the second year of their age. The investigation was fulfilled on 93 healthy males of mice taken into research at the age of 12 months. 45 of them formed an experimental group – they experienced during a year daily physical loads. The control group was composed of 48 healthy males who lived in usual vivarium conditions and didn’t experience physical loads. There were applied the following methods of investigation: biochemical, hematological and statistical. Mice under control while growing up were found to have gradual rise of intravascular thrombocytes’ activity. Regular daily physical loads between 12 and 24 months of life suppressed the rise of investigated mice’ intravascular thrombocytes’ activity connected with age. Regular physical loads are able to suppress in case of mice of the 2nd year of life rising with age inclination to the increase of thrombocyte aggregate formation in their blood.
Keywords
Thrombocytes; aggregation; ageing; mice; physical loads
Download this article as:| Copy the following to cite this article: Zavalishina S. Y, Vatnikov Y. A, Makurina O. N, Kulikov E. V, Sotnikova E. D, Parshina V. I, Rystsova E. O, Kochneva M. V, Sturov N. V. Diagnostical Appreciation of Physiological Reaction of intravascular Thrombocytes' Activity of two-Years-old Mice to Regular Physical Loads. Biomed Pharmacol J 2017;10(1). |
| Copy the following to cite this URL: Zavalishina S. Y, Vatnikov Y. A, Makurina O. N, Kulikov E. V, Sotnikova E. D, Parshina V. I, Rystsova E. O, Kochneva M. V, Sturov N. V. Diagnostical Appreciation of Physiological Reaction of intravascular Thrombocytes' Activity of two-Years-old Mice to Regular Physical Loads. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13820 |
Introduction
Age-specific changes can be met in the whole wildlife. They are mostly genetically conditioned mechanism (Amelina et al., 2009; Dontcov et al., 2010 ) of gradual weakening of organism’s functions leading finally to its inevitable death and to natural change of generations (Kiskun, 2008; Medvedev et al., 2012a). It was noted that different diseases (Medvedev et al., 2010d; Mitrokhina et al., 2014; Karar et al., 2015) and ontogenetic changes negatively influence functional features of thrombocyte hemostasis of mammals and men (Medvedev et al., 2005a; Kutafina et al., 2015b) often promoting thrombophilia formation in their organisms (Medvedev et al., 2004a; Vatnikov et al., 2015). Given phenomena have rather great significance in the process of different diseases’ progressing even in young years (Medvedev et al., 2010b; Sizov et al., 2015). Thrombocytes’ hyper functioning is especially evident traced at cardiovascular pathology (Simonenko et al., 2011c; Medvedev et al., 2013) met among men more often with ageing (Cary, 2012; Medvedev et al., 2012b). Because of the fact that the surface of activated thrombocytes is the basis for all hemostasis processes (Medvedev et al., 2004b) it becomes clear that at increasing of their ability to aggregation invivo risk of different vessels’ thrombosis sharply increases (Simonenko et al., 2010b; Medvedev et al., 2010). That’s why experimental search of available variants of blood platelets activity optimization in conditions of age-specific pathology (Medevedev et al., 2005c) and especially on the background of its appearance risk (Medvedev et al., 2016b; Zavalishina et al., 2016) is of great practical interest. As a variant of impact we thought to be perspective application of nonmedicinal means including very effective and popular ones – adequate physical practices (Medvedev et al., 2015a). They don’t have negative side effects and have already shown the ability to decrease to some extent thrombocyte activity at cardiovascular diseases (Gromnatskii et al., 2003; Medvedev et al., 2006). Their application decreased the danger of thrombosis coming what is one of leading factors of lifetime limitation at cardial pathology (Simonenko et al., 2007a; Purushothaman et al., 2014). At the same time some abilities of physical practices aren’t cleared up enough. We mean limitation field of age-specific thrombocyte activity strengthening in a mature healthy organism without any pathology signs. It’s rather convenient to solve the given problem in model conditions with the help of laboratory animals. That’s why the aim of the research was formulated as follows – to appreciate physical loads’ impact on rising intravascular thrombocyte activity of healthy mice during the second year of their life.
Materials and Methods
Fulfilled work was made in strict accordance with ethical principles established by the European convention about the defense of vertebral animals used for experimental and other scientific aims (adopted in Strasbourg on 18. 03. 1986 and confirmed in Strasbourg on 15. 06. 2006).
There were taken 93 healthy mice-males of 12-months’ age into the investigation. 45 of them composed experimental group and 48 composed control group.
The formation of experimental and control animal groups was made by casual getting mice out of the cages, where animals of the same age lived, after their darkening for the removal of the researcher’s subjective factor. Both experimental and control animals were healthy during the whole period of time preceded taking into investigation, were kept in similar conditions and haven’t taken part in any experiments before.
Animals of both groups were kept in vivarium in spacious cages (the area of cage’s floor for one animal was 299cm2). In one cage there were kept not more than 8 individuals. Cages were changed twice a week, Animals were removed into clean disinfected cages. The floor of cages was covered with litter of 5-10mm width (sawdust, wood chippings or felt turf) which was autoclaved before application at the temperature of 150-1800C. The litter was changed every day. Natural light was used; the temperature was kept at the level of 18-220C and relative humidity of 50-65%. Maximum allowed concentration of ammonia in vivarium was considered to be 0,01mg/l, of carbonic acid in the whole volume – 0,15%, at ventilation rate (volumes in an hour) – drawing out – 8, inflow – 10. Mice got fully rationed combined feed for laboratory animals PK-120 produced by the firm “Laboratorkorm” (Moscow, Russia). Water was in free access.
For the fulfillment of biochemical and hematological investigations animals’ blood was taken through a thick needle from caudal vein. The activity of plasma’s lipid peroxidation processes was appreciated according to the quantity of thiobarbituric acid-active products in it with the help of reagents’ set produced by the firm “Agat-Med” (Russia) and to the level of acylhydroperoxides (Chevari et al., 1991) taking into account plasma antioxidant activity (Volchegorskiy et al., 2000). The number of thrombocytes in blood was defined in Gorjaev’s cell. Intravascular thrombocyte activity was appreciated with the help of phase-contrast microscopy (Medvedev et al., 2009).
Experimental animals during a year experienced daily physical loads on horizontal treadmill TORNEO by the firm KETLER moving with the speed 5m/min. Animals were placed in one of the sections of a rectangular wooden framework placed on the treadmill and divided by wooden partitions into 3 parts for individual placement of an animal. On the first day the duration of loading was equal to 1min, then each day it became longer on 1min, till it reached 25minutes a day at its follow-up invariable duration during a day to the end of investigation (Pyabysheva, 2012). The appreciation of the common mice’ state in experimental and control groups was fulfilled daily at thrice-repeated registration of all the considered indices: at the beginning of investigation (at the age of 12 months), at the age of 18 months and at the age of 24 months. Statistical processing of the results was fulfilled by Student’s t-criteria.
Table 1: Biochemical and hematological indices of 2nd year old mice on the background of regular physical loads
| Indicators | Experimental group, M±m(n=45) | Control group, M±m (n=48) | ||||||
| 12 months | 18 months | 24 months | 12 months | |||||
| of plasma, D233/l ml | 1,53±0,015 | 1,56±0,014 | 1,59±0,019 | 1,52±0,018 | 1,60±0,024* | 1,95±0,033** | ||
| Thiobarbituric | ||||||||
| acid-products of | ||||||||
| plasma, mkmol/l | 3,59±0,012 | 3,62±0,016 | 3,66±0,021 | 3,61±0,022 | 3,80±0,016* | 4,22±0,042** | ||
| Antioxidant activity | ||||||||
| of plasma, % | 32,8±0,33 | 32,4±0,29 | 32,2±0,37 | 32,6±0,24 | 30,7±0,32* | |||
| Thrombocytes- | ||||||||
| discocytes, % | 78,9±0,22 | 78,2±0,14 | 77,9±0,19 | 79,4±0,18 | 77,2±0,15* | |||
| Sum of thrombocytes’ | ||||||||
| active forms, % | 21,1±0,18 | 21,8±0,15 | 22,1±0,16 | 20,6±0,14 | 22,8±0,19* | 29,6±0,17** | ||
| Thrombocytes’ | ||||||||
| number in | ||||||||
| aggregates, % | 4,9±0,08 | 4,9±0,07 | 5,1±0,09 | 4,8±0,12 | 4,9±0,05* | |||
| Number of little | ||||||||
| aggregates | ||||||||
| (in 100 free | ||||||||
| thrombocytes) | 3,6±0,10 | 3,7±0,09 | 3,8±0,12 | 3,5±0,07 | 3,6±0,09* | |||
| Number of medium | ||||||||
| and large aggregates | ||||||||
| (in 100 free | ||||||||
| thrombocytes) | 0,14±0,006 | 0,15±0,005 | 0,14±0,006 | 0,13±0,008 | 0,17±0,004* | 0,38±0,003** | ||
Results
Both experimental and control mice before the beginning of investigation showed no differences in all the considered indices. While ageing control animals were noted to have gradual increase of acylhydroperoxides’ and thiobarbituric acid-products’ quantity in plasma at the decrease of its antioxidant activity. At the same time experimental mice during investigation used to show stable level of plasma lipid peroxidation and its antioxidant protectability. So, at the age of 24 months they had acylhydroperoxides at the level 1,59±0,019 D233/1ml , thiobarbituric acid-active products – 3,66±0,021mkmol/l and at the value of plasma antioxidant activity – 32,2±0,37%. Control mice of 24months’ age had the following considered indices – 1,95±0,033 D233/1 ml, 4,22±0,042 mkmol/l and 26,2±0,27% correspondingly.
Compared at the start of investigation levels of thrombocytes-discocytes in the blood of mice from both groups began while ageing to differ evidently – in control group they decreased on 12,8% at the increase of thrombocytes’ active forms sum to 29,6±0,17%. The quantity of small and large thrombocyte aggregates in control mice’ blood during investigation period increased on 38,6% and 65,8% correspondingly. And the number of thrombocytes included into aggregates increased on 18,6% in case of control animals during the second year of life.
Conducted regular physical loads were accompanied in experimental group of mice by stability of not high intravascular thrombocyte activity (table). Discocytes’ quantity in bloodstream of these animals at the age of 24 months was equal to 77,9±0,19% at not large total quantity of blood platelets’ active forms (22,1±0,16%) (Figure). It provided invariably not high level of freely circulating aggregates of different sizes in their blood at not large thrombocytes’ involvement into them.
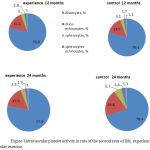 |
Figure 1: Intravascular platelet activity in rats of the second year of life, experiencing regular exercise.
|
Discussion
In previous investigations it was noted that regular physical loads are able in pathology conditions to influence positively many organism’s parameters including thrombocyte hemostasis (Gromnatskii et al., 2003; Medvedev et al., 2013). At the same time, potential of their impact in conditions of full health on coming with age changes of thrombocytes’ activity are still examined rather poorly (Shitikova, 2010; Kutafina et al., 2015a). In order to fill this gap in our scientific knowledge we fulfilled in experimental conditions the appreciation of regular moderate physical loads’ impact on the state of intravascular thrombocyte activity. The work was fulfilled with the help of mice of the 2nd year of life. This age was chosen because at this stage of mice’ ontogenesis many age-specific changes gradually appear and increase (Medvedev et al., 2016b).
The animals of the control group were noted to have gradual increase of lipid peroxidation activity in plasma. It is known that it, with the help of some mechanisms, is able to strengthen intravascular thrombocyte aggregation. One of these mechanisms is, without any doubt, stimulation of Willybrand’s factor production in vessels which is rather often met with age and is sometimes the leading cause of thrombophilia formation. Besides, on the background of age-specific peroxidation strengthening in plasma we had obligate depression increase of vascular antiaggregants’ synthesis – prostacyclin and nitric oxide (Simonenko et al., 2010a; Medvedev et al., 2016a). Judging by the increase of thrombocytes aggregants’ quantity in control mice’ blood on the surface of their thrombocytes there was age-specific gradual increase of thrombocyte receptors’ number and activity towards constantly present in blood physiological aggregation inductors – collagen, thrombone, ADF and participant of the given process – fibrinogen (Simonenko et al., 2007b). This, in its turn, inevitably led in case of control animals to strengthening in thrombocytes of their aggregation realization mechanisms activated under the impact of strong and weak inductors.In this context it will be correct to connect the growth of control animals’ thrombocyte aggregation in response to strong inductors with activity rise of thrombocyte phospholipase C, synthesis strengthening in thrombocytes of diacylglicerol and protein kinase C , rise of proteins’ phospholirirovation and their contractile system (Medvedev et al., 2005b). These changes in mice’ thrombocytes during the 2nd year of life inevitably led to strengthening of Ca2+ supply into them contributing to more evident actomyosin reduction in the process of thrombocytes’ activation by strong inductors (Simonenko et al., 2011b). There is no doubt that strengthening of thrombocyte reaction of control animals on weak inductors is mostly connected with activity rise of thrombocyte phospholipase A2 actively evolving arachidonic acid out of thrombocytes’ phospholipids (Medvedev et al., 2010c; Simonenko et al., 2011a), what leads to the intensification of the synthesis in them of a mighty aggregation stimulator – thromboxane A2 (Medvedev et al., 2015b). Summarizing literature data we can say that increase of thrombocyte aggregates’ number in control mice’ blood while ageing and decrease of discoid thrombocytes points not only at progressive increase of thrombocyte readiness to participation in hemostasis (Burnier et al., 2009; Safdar et al., 2015), but also at the stimulation from their side of all the rest hemostatical mechanisms (Medvedev et al., 2008; Garg et al., 2015; Jadhav et al., 2015).
Experimental mice experiencing during the 2nd year of life regular daily physical loads were noted to keep optimal thrombocyte activity. Reached effect was evidently possible as the result of maintaining on the optimal level of factors stimulating thrombocytes’ aggregation and mechanisms realizing it in case of experimental mice (in plasma, in thrombocytes’ membranes and thrombocyte cytoplasm). So, experimental mice during investigation were noted to keep rather high activity of plasma antioxidant system effectively suppressing lipids’ peroxidation processes in it. This minimized its negative impact on surface thrombocytes’ structures and vascular endothelium. Because of that having regular physical loads mice kept stable not large plasma level of Willybrand’s factor and functionally enough production of vascular antiaggregants – prostacyclin and nitric oxide (Dontcov et al., 2010). Low level of thrombocyte aggregates in experimental mice’ blood pointed at maintaining of the optimal state of their receptor and postreceptor mechanisms of thrombocytes’ functioning (Kutafina et al., 2015a). So, on experimental animals’ thrombocytes, without any doubt, was kept not high density and not large activity of thrombocyte receptors to ADF, collagen, thrombin and fibrinogen (Simonenko et al., 2007b). At the same time stabilization of not high thrombocyte activity was provided in case of experimental animals by keeping at the level near to the initial one of the activity of impact mechanisms on thrombocytes of strong and weak aggregation inductors. In this connection we can speak about experimental mice’ keeping not high activity of phospholipase C and proteinkinase C and not large intensity of proteins’ phospholirirovation of contractile thrombocytes’ system (Medvedev et al., 2005b). It led in these mice’ thrombocytes to supporting of stable not high production of diacylglicerol and inositolthreephosphat. It provided minimum necessary supply of Ca2+ from the depot into their cytoplasm suppressing in such a way the evidence of actomyosin reduction (Simonenko et al., 2011b). It became clear that experimental mice’ thrombocytes also kept not high phospholipase A2 activity. It provided evolving of physiologically minimum quantity of arachidonic acid out of their membranes’ phospholipids providing optimal level of thromboxane A2 synthesis (Medvedev et al., 2013; Medvedev et al., 2015b).
Conclusion
Healthy mice at the age of more than 12 months are noted to have gradual strengthening of thrombocytes’ aggregation ability in vivo. Regular daily physical loads of mice between 12 and 24 months of their life keep intravascular thrombocytes’ activity at the level near to the initial one suppressing its age-specific strengthening.
Conflict of Interest
No Conflict of interest to declare.
References
- Amelina, I.V. and Medvedev, I.N. Transcriptional activity of chromosome nucleolar organizing regions in population of Kursk region. Bulletin of Experimental Biology and Medicine. 2009; 147(6) : 730-32.
- Burnier, L., Fontana, P, Kwak, B.R. and Angelillo-Scherrer, A. Cell-derived microparticles in haemostasis and vascular medicine. Haemost. 2009; 101 : 439-51.
- Cary, N. Epigenetics (Phoenix, Rostov -on-Don) 2012 : 349.
- Chevari, S., Andyal, T. and Strenger, J. Determination of antioxidant blood parameters and their diagnostic value in the elderly. Laboratory work. 1991; 10 : 9-13.
- Dontcov, V.N., Krut’ko, V.N., Trukhanov, A.I. Anti-aging medicine: fundamentals. Moscow: Krasnodar, 2010 : 680.
- Garg, R., Aggarwal, S., Kumar, R. and Sharma, G. Association of atherosclerosis with dyslipidemia and co-morbid conditions: A descriptive study. J Nat Sc Biol Med. 2015; 6: 163-68.
- Gromnatskii, N.I. and Medvedev I.N. Non-pharmacological correction of impaired platelet hemostasis in hypertensive patients with metabolic syndrome. Klinicheskaia meditsina. 2003; 81(4) : 31-4.
- Jadhav, G.R., Shah, D. and Raghvendra, S.S. Autologus Platelet Rich Fibrin aided Revascularization of an immature, non-vital permanent tooth with apical periodontitis: A case report. J Nat Sc Biol Med. 2015; 6: 224-25.
- Karar, T., Elfaki, E.M. and Qureshi, S. Determination of the serum levels of troponin I and creatinine among Sudanese type 2 diabetes mellitus patients. J Nat Sc Biol Med. 2015; 6: S80-4.
- Kiskun, A.A. Biological age and aging: the possibility of identifying and correcting the path. Moscow: GEOTAR Media, 2008 : 976.
- Kutafina, N.V. and Medvedev, I.N. Platelet aggregation clinically healthy persons of the second coming of age living in the Kursk region. Advances in gerontology = Uspekhi gerontologii / Rossiiskaia akademiia nauk, Gerontologicheskoe obshchestvo. 2015; 28(2) : 321-25
- Kutafina, N.V. and Medvedev, I.N. Platelet Aggregation in Clinically Healthy Persons of the Second Coming-of-Age Living in the Kursk Oblast. Advances in Gerontology. 2015; 5(4) : 267-70.
- Medvedev, I.N., Gromnatskii, N.I., Golikov, B.M., Al’-Zuraiki, E.M. and Li, V.I. Effects of lisinopril on platelet aggregation in patients with arterial hypertension with metabolic syndrome. Kardiologiia. 2004; 44(10) : 57-9.
- Medvedev, I.N., Gromnatskii, N.I., Volobuev, I.V., Dement’ev, V.I. and Storozhenko, M.V. Thrombocytic hemostasis in hypertensive patients with metabolic syndrome and its correction with lovastatin. Klinicheskaia meditsina. 2004; 82(10) : 37-41.
- Medvedev, I.N. and Gromnatskii, N.I. Correction of thrombocyte hemostasis and biological age reduction in metabolic syndrome. Klinicheskaia meditsina. 2005; 83(8) : 54-7.
- Medvedev, I.N. and Gromnatskii, N.I. Effect of amlodipine on intravascular thrombocyte activity in patients with arterial hypertension and metabolic syndrome Klinicheskaia meditsina. 2005; 83(2) : 37-9.
- Medvedev, I.N. and Gromnatskii, N.I. The influence of nebivolol on thrombocyte aggregation in patients with arterial hypertension with metabolic syndrome. Klinicheskaia meditsina. 2005; 83(3) : 31-3.
- Medvedev, I.N. and Gromnatskii, N.I. The influence of hypocaloric diet on thrombocyte rheology in patients with metabolic syndrome. Klinicheskaia meditsina. 2006; 84(3) : 49-52.
- Medvedev, I.N. and Kumova, T.A. Reduced platelet aggregation in losartan-treated patients with arterial hypertension and metabolic syndrome. Russian Journal of Cardiology. 2008; 1 : 40-2.
- Medvedev, I.N., Savchenko, A.P., Zavalishina, S.Yu. and Krasnova, E.G. Methodological approaches to the study of the rheological properties of blood in various states. Russian Journal of Cardiology. 2009; 5 : 42-5.
- Medvedev, I.N. and Skoryatina, I.A. Platelet hemostasis dynamics in simvastatin- treated patients with arterial hypertension and dyslipidemia. Russian Journal of Cardiology. 2010; 1(81) : 54-8.
- Medvedev, I.N. and Savchenko, A.P. Platelet activity correction by regular physical training in young people with high normal blood pressure. Russian Journal of Cardiology. 2010; 2(82): 35-40.
- Medvedev, I.N. and Skoriatina, I.A. Effect of lovastatin on adhesive and aggregation function of platelets in patients with arterial hypertension and dyslipidemia. Klinicheskaia meditsina. 2010; 88(2) : 38-40.
- Medvedev, I.N., Lapshina, E.V. and Zavalishina, S.Yu. Activity of platelet hemostasis in children with spinal deformities. Bulletin of experimental biology and medicine. 2010; 149(5) : 645-46.
- Medvedev, I.N. and Amelina, I.V. An association between human morphological phenotypical characteristics and the activity of chromosomal nucleolar organizer regions in the interphase cell nucleus in the population of indigenous people of Kursk region. Morfology. 2012; 142(4) : 87-91.
- Medvedev, I.N. and Skoriatina, I.A. Dynamics of microrheologic properties of erythrocytes in patients with arterial hypertension and dyslipidemia treated with atorvastatin. Klinicheskaia meditsina. 2012; 90(6) : 42-5.
- Medvedev, I.N. and Skoryatina, I.A. Fluvastatin effects on blood cell aggregation in patients with arterial hypertension and dyslipidemia. Cardiovascular Therapy and Prevention. 2013; 12(2) : 18-24.
- Medvedev, I.N., Savchenko, A.P. and Kiperman, Ya.V. Dynamics of the Intravascular Activity of Platelets in Young Men with High Normal Blood Pressure Regularly Practicing Physical Activity. Biology and Medicine (Aligarh). 2015; 7:1 BM-069-15.
- Medvedev, I.N. and Skoryatina, I.A. Aggregation properties of blood cells and vascular control over them in patients with arterial hypertension and dyslipidemia. Russian Journal of Cardiology. 2015; 4(120) : 18-22.
- Medvedev, I.N. and Zavalishina, S.Yu. Platelet Activity in Patients With Third Degree Arterial Hypertension and Metabolic Syndrome. Kardiologiia. 2016; 56(1) : 48.
- Medvedev, I.N. Dynamics of violations of intravascular platelet activity in rats during the formation of metabolic syndrome using fructose models. Problems of nutrition. 2016; 85(1) : 42-6.
- Mitrokhina, N.V., Vatnikov, Y.A., Sotnikova, E.D. and Kulikov, E.V. Assessment of the risk of osteosarcoma recurrence in canine long bone replants. European Journal of Physical and Health Education. 2014; 6 : BM-006-14.
- Purushothaman, S., Salmani, D., Prarthana, K.G., Bandelkar, S.G. and Varghese, S. Study of ECG changes and its relation to mortality in cases of cerebrovascular accidents. J Nat Sc Biol Med. 2014; 5:434-36.
- Ryabysheva, S.S. Hronotromnaya heart function of rats exposed to various motor regimes. Young scientist. 2012; 1(1) : 69-72.
- Safdar, A., Shaaban, H., Tibayan, R., Miller, R., Boairdo, R. and Guron, G. The clinical efficacy of using autologous platelet rich plasma in hip arthroplasty: A retrospective comparative study. J Nal Sc Biol Med. 2015; 6: 49-55.
- Shitikova, A.S. Thrombocytopathy congenital and acquired. St. Petersburg, 2008 : 320.
- Simonenko, V.B., Medvedev, I.N., Mezentseva, N.I. and Tolmachev, V.V. The antiaggregation activity of the vascular wall in patients suffering from arterial hypertension with metabolic syndrome. Klinicheskaia meditsina. 2007; 85(7) : 28-30.
- Simonenko, V.B., Medvedev, I.N. and Tolmachev, V.V. Comparative evaluation of the influence of sulfhydryl and phosphate ACE inhibitors on thrombocyte aggregation in patients suffering from arterial hypertension with metabolic syndrome. Klinicheskaia meditsina. 2007; 85(4) : 24-7.
- Simonenko, V.B., Medvedev, I.N. and Tolmachev, V.V. Effect of irbesartan on the function of hemocoagulative component of hemostasis in patients with arterial hypertension during metabolic syndrome. Klinicheskaia meditsina. 2010; 88(6) : 27-30.
- Simonenko, V.B., Medvedev, I.N. and Kumova, T.A. Pathogenetic aspects of hypertension in case of metabolic syndrome. Voenno-meditsinskiǐ zhurnal. 2010; 331(9) : 41-4.
- Simonenko, V.B., Medvedev, I.N. and Tolmachev, V.V. Dynamics of primary hemostasis activity in patients with arterial hypertension and metabolic syndrome treated with candesartan. Klinicheskaia meditsina. 2011; 89(3) : 35-8.
- Simonenko, V.B., Medvedev, I.N. and Gamolina, O.V. Primary hemostasis activity in patients with arterial hypertension and impaired glucose tolerance treated with trandolapril. Klinicheskaia meditsina. 2011; 89(2) : 29-31.
- Simonenko, V.B., Medvedev, I.N. and Tolmachev, V.V. Pathogenetic aspects of arterial hypertension in metabolic syndrome. Klinicheskaia meditsina. 2011; 89(1) : 49-51.
- Sizov, A.A., Zavalishina, S.J. Russian Criminal Legislation in Prevention of Sexually Transmitted Diseases in the Territory of the Russian Federation. Biology and Medicine (Aligarh). 2015; 7 (5): BM-142-15, 5 pages.
- Vatnikov, Y.A., Sakhno, N.V., Sotnikova, E.D., Kulikov, E.V., Parshina, V.I. and Troshina, N.I. Clinical control of packed RBC transfusion in acute surgical pathology such as gastric dilation and volvulus in dogs. Biomedical and Pharmacology Journal. 2015, 8(2) : 711-17.
- Volchegorskiy, I.A., Dolgushin, I.I., Kolesnikov, O.L. and Tseilikman, V.E. Experimental modeling and laboratory evaluation of adaptive reactions of the organism. Chelyabinsk, 2000 : 167.
- Zavalishina, S.Yu., Kutafina, N.V., Vatnikov, Yu.A., Makurina, O.N., Kulikov, E.V., Rystsova, E.O., Gurina, R.R. and Sotnikova, E.D. Platelet-Activity Dependence on the Age of Rats with Experimental Dyslipidemia. Biol Med (Aligarh). 2016; 8: 326. doi: 10.4172/0974-8369.1000326.







