Manuscript accepted on :
Published online on: 23-11-2015
Plagiarism Check: Yes
Robles-Piedras Ana Luisa1,2, Fuentes-Noriega Inés3, Romano-Moreno Silvia4, Mancilla-Urrea Eduardo5, Domínguez-Ramírez Adriana Miriam6
1Doctorado en Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana Unidad Xochimilco, Calzada del Hueso 1100, Col. Villa Quietud, Delegación Coyoacán, México, D.F. México. 2Cuerpo Académico de Farmacia Clínica. Área Académica de Farmacia, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Circuito Ex-Hacienda La Concepción, Km. 1.5, San Agustín Tlaxiaca, Hidalgo. México. C.P. 42160 3Departamento de Farmacia, Facultad de Química, Universidad Nacional Autónoma de México, Conjunto “E”, Laboratorio 113, Paseo de la Investigación Científica s/n , Ciudad Universitaria, Delegación Coyoacán, México, D.F. México. C.P. 04510 4Laboratorio de Biofarmacia y Farmacocinética, Facultad de Ciencias Químicas, Universidad Autónoma de San Luis Potosí, Avenida Dr. Manuel Nava no. 6, Zona Universitaria, San Luis Potosí, SLP. México. C.P.78240 5Departamento de Nefrología, Instituto Nacional de Cardiología “Ignacio Chávez”, Juan Badiano no. 1, Col. Secc. XVI, Delegación Tlalpan, México, D.F. México. C.P. 14080 6Laboratorio de Farmacocinética, Departamento de Sistemas Biológicos, Universidad Autónoma Metropolitana Unidad Xochimilco, Calzada del Hueso 1100, Col. Villa Quietud, Delegación Coyoacán, México, D.F. México. C.P. 04960.
DOI : https://dx.doi.org/10.13005/bpj/576
Abstract
Tacrolimus is an immunosuppressive agent that has been proven safe and effective for the prevention of graft rejection after organ transplantation. Between 2008 and 2010, the FDA approved several generic formulations of TAC. In Mexico, the first generic TAC product was approved in 2006 and currently in the domestic pharmaceutical market there are >5 interchangeable generic products. The objective of this study was to compare TAC blood concentrations as well as serum creatinine and creatinine clearance as a measure of renal function found in patients with kidney transplantation with administration of the innovator (Prograf) and the subsequent change to the generic product. The analysis done showed that after the change to generic product, 69% of the patients required a dose adjustment of Tacrolimus (p<0.05). Mean values of serum creatinine exhibited an increase of 3% (p<0.05). Creatinine clearance also showed a decrease after conversion from Prograf to the generic product (p<0.05). The information obtained from patients treated with innovator drugs and generics constitute an important clinic tool, the data obtained is greatly valued, especially on the absence of previous information related to the conversion from Prograf to a generic Tacrolimus drug in Mexican receptors from renal transplants.
Keywords
Conversion; Generic; Kidney; Prograf; Tacrolimus; Transplant
Download this article as:| Copy the following to cite this article: Luisa R. P. A, Inés F. N, Silvia R. M, Eduardo M. U, Miriam D. R. A. Generic Substitution of Tacrolimusi De Novo Mexican Renal Transplant Recipients. A Single Center Experience.. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Luisa R. P. A, Inés F. N, Silvia R. M, Eduardo M. U, Miriam D. R. A. Generic Substitution of Tacrolimusi De Novo Mexican Renal Transplant Recipients. A Single Center Experience. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1380 |
Introduction
Tacrolimus (TAC) is an immunosuppressive agent that has been proven safe and effective for the prevention of graft rejection after organ transplantation. In 1994 in the United States, it gained approval for use in liver transplantation, and about 3 years later was approved for prevention of acute renal transplant rejection1. Between 2008 and 2010, the Food and Drug Administration (FDA) approved several generic formulations of TAC2. In Mexico, the first generic TAC product was approved in 2006 and currently in the domestic pharmaceutical market there are more than five interchangeable generic products. When speaking of generic drugs, one of the most important concepts is that of bioequivalence. Bioequivalence is the quality that exhibits a generic and innovative drug with the same active ingredient and that are equivalent and interchangeable from the viewpoint of quality, safety, and efficacy. When two drugs are equivalent in the rate and amount at which the active drug is absorbed and reaches the site where the effect is produced, the two are therapeutically equivalent and can be used interchangeably.
To demonstrate that a pharmaceutical product is interchangeable with the innovator product requires that bioequivalence studies show that the 90% Confidence interval (90% CI) for the Area Under the Curve of the drug concentration (AUC) profile vs. time and that maximal serum concentration (Cmax) falls within the acceptance limits of 80-125% compared with the values of the reference product (90-111% for drugs with a narrow therapeutic index)3. Specifically, the acceptable variation is dependent on the type of drug involved; thus, in general, AUC values (amount of the bioavailable drug) may differ from each other by up to 45%, and possible fluctuations may expose the patient to different situations whether or not the generic-in-question has an AUC of 80% or 125% of the original drug. This implies that the patient could be exposed to an increased concentration of the drug with the potential risk for toxicity, or could be exposed to a sudden decrease in concentration with the risk of treatment failure.
Despite this, the variability margins allowed do not ensure bioequivalence for drugs with a narrow therapeutic window, such as immunosuppressive agents. In immunosuppressive drugs and in order to demonstrate the bioequivalence of generic drugs, exhaustive and rigorous studies must be performed in patients with transplantation to prove their effectiveness, as have been conducted in other countries4,5.
However, in Mexico these studies have only been performed in healthy volunteers, and under these specifications, there is no requirement that generic products demonstrate bioequivalence in the target population, because once interchangeability has been established, therapeutic equivalence is assumed in generic drugs4. There is evidence that even when bioequivalence criteria are met, some generic drugs differ with respect to the innovator product6,7. Additionally, it has been reported that generic formulations containing TAC marketed in Mexico and other countries do not meet criteria for biopharmaceutical quality8,9 . Within this context, the objective of this study was to compare TAC blood concentrations found in patients with kidney transplantation with administration of the innovator product (Prograf®, Janssen-Cilag. Mexico, D.F.) and the subsequent change to the generic product, and in addition performed a correlation between serum creatinine and creatinine clearance as a measure of renal function.
Patients and Methods
We conducted a retrospective-prospective study that included 29 adult patients undergoing renal transplantation at the National Institute of Cardiology in Mexico City. The immunosuppressive regimen consisted of a triple scheme based on TAC, mycophenolate mofetil, and prednisone. The initial dose of TAC was calculated at 0.13 mg/Kg, which was subsequently adjusted to achieve blood concentration levels of 10-15 ng/mL during the first 3 months after transplantation and levels of 5-8 ng/mL thereafter. All patients received Prograf twice daily for the first 6 months after transplantation and subsequent conversion into the generic product. Conversion was carried out with the same dose of TAC and at least 1 week passed prior to taking the first levels of TAC with the generic drug. Throughout the review of clinical records and follow-up of patients in the outpatient clinic, the following data were collected:
TAC blood concentration before and after the change between products based on pre- vs. postconversion concentrations at the same dose. Serum creatinine and creatinine clearance by Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI)10 criteria were based on values prior to conversion vs. those of the last follow-up. The relationship between TAC concentration and the daily dose (C/D) before and after conversion was also calculated. TAC levels were measured by the chemiluminescence immunoassay method (ARCHITECT SYSTEM; Abbott, Abbott Park IL, USA).
Statistical Methods
Results are presented as mean (± Standard deviation [SD]). The paired Student t test was used to analyze mean TAC levels, serum creatinine levels, creatinine clearance, and the daily TAC dose, as well as the relationship between TAC concentration and the dose administered (such as C/D ratios) before and after the patient’s change-over to the generic product. The threshold of statistical significance was set at 95% (= 0.05).
Results
The study included 29 patients (16 men and 13 women), mean age 31 ± 11 years (range, 17-63 years), 82.8% received living related donor graft, 10.3% unrelated living donor, and 6%, deceased donor. Follow-up time was 149 ± 74 days for use with Prograf and 370 ± 15 days for use with generic drugs. Two patients experienced acute cellular rejection prior to the switch and were treated with three methylprednisolone boluses with total recovery of graft function.
Table 1 presents the values of the TAC dose (mg/kg/day), trough concentration, C/D ratio, and values of serum creatinine (mg/dL) and creatinine clearance (mL/min) before and after the change of Prograf to the generic drug. The mean daily TAC dose required to maintain trough concentration (5-8 ng/mL) was 0.098 ± 0.05 mg/kg/day for Prograf and 0.088 ± 0.05 mg/k/day for the generic product (p < 0.05).
Table 1: Tacrolimus dose, trough concentration, C/D ratio, and values of serum creatinine (mg/dL) and creatinine clearance (mL/min) before and after the change of Prograf to the generic drug.
| Prograf® | Tacrolimus Generic* | |
| Follow-up(days) | 149±74 | 370±151 |
| Dose (mg/kg/day) | 0.098±0.05 | 0.088±0.05 |
| Ctrough (ng/mL) | 8.56±2.4 | 8.28±2.9 |
| C/D ratio | 103±63.2 | 107±66.9 |
| Serum creatinine (mg/dL) | 1.03±0.24 | 1.08 ±0.23 |
| Creatinine clearance (mL/min)
|
86.4±20.3 | 81.9±14.4 |
*post-conversion
After conversion, TAC doses required modification as follows: reduction in 48% (n = 14), 31% (n = 9) continued with the same dose, and in 21%, this increased (n = 6). The value of the mean TAC trough concentration before and after the switch between products is illustrated in Figure 1. While marked variation was observed along monitoring, statistical analysis demonstrated no significant difference (p > 0.05). The mean value of the C/D ratio for Prograf was 103 ± 63.4 and 107 ± 66.8 [(ng/mL)/(mg/kg/day)] (mean ± SD) for generic products (p > 0.05). The mean serum creatinine observed with the use of Prograf rose from 1.08 ± 0.34 mg/dL to 1.11 ± 0.26 mg/dL (p < 0.05). Additionally, average creatinine clearance exhibited a statistically significant decrease (p < 0.05) after conversion from Prograf (87.9 ± 18.7 mL/min) to generic product (82.9 ± 13.5 mL/min). Mean creatinine clearance at pre- and post-switch is shown in Figure 2.
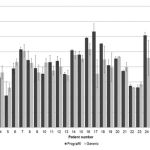 |
Figure 1: Mean ± Standard deviation (SD) of tacrolimus trough concentration pre- and post-switch from Prograf to generic product. |
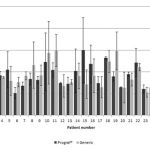 |
Figure 2: Graph of mean creatinine clearance ± Standard deviation (SD) (on left y axis) in 29 patients, pre- and post-switch from Prograf to generic tacrolimus. |
Discussion
Currently in Mexico, there are products available that contain multiple generic TAC; however, although the use of various products in clinical practice differs with regard to the therapeutic results observed, these, to our knowledge, remain undocumented. In our study, all patients received Prograf for 6 months prior to conversion; thus, it was possible to evaluate patients administered the brand-name drug and then when the patients converted to a generic product at the same dose, taking each patient as his/her own control. The generic product change resulted in an increase in the mean through concentration of TAC in 12 patients, and this decreased in 16 patients, but the difference between mean value of drug levels before and after the change between products was not found to be statistically significant (p > 0.05). This result agrees with that obtained by other authors who evaluated the impact of the conversion from Prograf to the generic product, which reported only minor changes in the minimal concentration of TAC after conversion. In other prospective studies, results are also similar11,12.
Our analysis showed that after the switch to the generic product, 69% of patients required a dose adjustment to maintain the drug concentration within the therapeutic range. These results demonstrated statistically significant differences (p < 0.05), however this could be expected because in general, small dose changes are made in the clinical management of patients with transplant2,4,12 . In the C/D ratio, substitution with generics resulted in an increase of 4% (p > 0.05) and wide interindividual variability; thus, measurement of TAC blood concentrations at the time of Prograf replacement by a generic product is essential in therapeutics, because small changes in dose can result in large changes in these levels.
In the population studied and with the use of generic drugs, no acute rejection episodes occurred; however, mean values of serum creatinine exhibited an increase of 3% (p < 0.05). Creatinine clearance also showed a decrease that proved to be statistically significant after conversion from Prograf to the generic product (p < 0.05). Both observations can compromise patient and graft survival in both the short and long term. High intra- and intersubject variability in immunosuppressant drug exposure is known to have serious consequences in recipients of solid organ transplants, such as increased rates of rejection and worse, graft loss13,14. This variability is principally the result of genetic polymorphisms, ethnic background, co-morbid diseases, and interactions with other concomitantly administered drugs, and is determined by the individual patient’s characteristics and will also occur with other formulations, including the innovator drug15. Nonetheless, the problem in Mexico is even more complex, because there are now multiple generic products that patients acquire to take alternately; therefore, the potential for variability is amplified because such formulations are not bioequivalent to each other16.
Several bioequivalence studies with immunosuppressive drugs have been questioned by a number of researchers because they do not reflect conditions applicable to clinical practice. This is because they are conducted in healthy volunteers and at a single dose under fasting conditions, when in fact the drugs are administered chronically, with meals and in the majority of cases, twice daily4,17-19. Moreover, this type of study does not assess the immunosuppressive capability of these drugs. Thus, we think that the information obtained in studies such as this comprises an important decision-making tool in clinical practice.
We consider that our study has several limitations, such as sample size and the short follow-up after conversion to generic drug (approximately 1 year), because of which we were not able to analyze a particular generic brand because the majority of the patients received the drug through the Mexican Institute of Social Security and a variability of brands required follow-up. However, we believe these results are valuable because each patient served as his/her own control to conversion.
Conclusions
To our knowledge, there is no previous published data on the impact of replacing innovator product Prograf with the TAC generic in Mexican patients with renal transplantation. It is necessary to carry out more studies of this type in Mexico that include a larger number of patients, attempting to separate study populations by generic drug brands and primarily, to perform proper long-term monitoring in order to truly assess the impact of the use of generic drugs on the survival of patients and grafts.
References
- Bowman, L.J., Brennan, D.C. The role of tacrolimus in renal transplantation. Expert. Opin. Pharmacol. Ther., 2008; 9(4): 635-43.
- Ensor, C.R., Trofe-Clark, J., Gabardi, S., McDevitt-Potter, L.M., Shullo, M.A. Generic maintenance immunosuppression in solid organ transplant recipients. Pharmacotherapy, 2011; 31(11): 1111-29.
- Secretaría de Salud “Norma Oficial Mexicana NOM-177-SSA1-2013. Que establece las pruebas y procedimientos para demostrar que un medicamento es intercambiable. Requisitos a que deben sujetarse los terceros autorizados que realicen las pruebas”: Diario Oficial de la Federación 6 mayo 2013, México.
- Alloway, R.R., Sadaka, B., Trofe-Clark, J., Wiland, A., Bloom, R.D. A randomized pharmacokinetic study of generic tacrolimus versus reference tacrolimus in kidney transplant recipients. Am. J. Transplant., 2012; 12(10): 2825-31.
- Bloom, R.D., Trofe-Clark, J., Wiland, A., Alloway, R.R. A randomized, crossover pharmacokinetic study comparing generic tacrolimus vs. the reference formulation in subpopulations of kidney transplant patients. Clin. Transplant., 2013; 27(6): E685-93.
- Alloway, R.R., Isaacs, R., Lake, K., Hoyer, P., First, R., Helderman, H., Bunnapradist, S., Leichtman, A., Bennett, M.W., Tejani, A., Takemoto, S.K. Report of the American Society of Transplantation conference on immunosuppressive drugs and the use of generic immunosuppressants. Am. J. Transplant., 2003; 3(10): 1211-5.
- Kahan, B.D. Considerations concerning generic formulations of immunosuppressive drugs. Transplant. Proc., 1999; 31(3): 1635-41.
- Esquivel, A., González-Ramírez, R., Alberú, J., Gracida, C., Medeiros, M., Castañeda-Hernández, G. Comparison of dissolution properties of 2 enteric-coated formulations containing mycophenolate sodium: Myfortic vs Femulan. Transplant. Proc., 2010; 42(1): 353-6.
- Petan, J.A., Undre, N., First, M.R., Saito, K., Ohara, T., Iwabe, O., Mimura, H., Suzuki, M., Kitamura, S. Physiochemical properties of generic formulations of tacrolimus in Mexico. Transplant. Proc., 2008; 40(5): 1439-42.
- CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. Available in http://www.qxmd.com/calculate-online/nephrology/ckd-epi-egfr.
- Momper, J.D., Ridenour, T.A., Schonder, K.S., Shapiro, R., Humar, A., Venkataramanan, R. The impact of conversion from Prograf to generic tacrolimus in liver and kidney transplant recipients with stable graft function. Am. J. Transplant., 2011; 11(9): 1861-7.
- McDevitt-Potter, L.M., Sadaka, B., Tichy, E.M., Rogers, C.C., Gabardi, S. A multicenter experience with generic tacrolimus conversion. Transplantation, 2011; 92(6): 653-7.
- Borra, L.C., Roodnat,J.I., Kal, J.A., Mathot, R.A., Weimar, W., van Gelder, T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol. Dial. Transplant., 2010; 25(8): 2757-63.
- Pollock-Barziv, S.M., Finkelstein, Y., Manlhiot, C., Dipchand, A.I., Hebert, D., Ng, V.L., Solomon, M., McCrindle, B.W., Grant, D. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr. Transplant., 2010; 14(8): 968-75.
- Christians, U., Klawitter, J., Clavijo, C.F. Bioequivalence testing of immunosuppressants: concepts and misconceptions. Kidney Int. Suppl., 2010; (115): S1-7.
- Harrison, J.J., Schiff, J.R., Coursol, C.J., Daley, C.J., Dipchand, A.I., Heywood, N.M., Keough-Ryan, T.M., Keown, P.A., Levy, G.A., Lien, D.C., Wichart, J.R., Cantarovich, M. Generic immunosuppression in solid organ transplantation: a Canadian perspective. Transplantation, 2012; 93(7): 657-65.
- Van Gelder, T., ESOT Advisory Committee on Generic Substitution. European Society for Organ Transplantation Advisory Committee Recommendations on Generic Substitution of Immunosuppressive Drugs. Transpl. Int., 2011; 24(12): 1135-41.
- van Gelder, T., Gabardi, S. Methods, strengths, weaknesses, and limitations of bioequivalence tests with special regard to immunosuppressive drugs. Transpl. Int., 2013; 26(8): 771-7.
- Benet, L.Z. Relevance of pharmacokinetics in narrow therapeutic index drugs. Transplant. Proc., 1999; 31(3): 1642-4.







