Alok K. Pareek*, P. E. Joseph and Daya S. Seth
School of Chemical Sciences, Department of Chemistry, ST. John’s College, Agra - 282002 India.
Abstract
Pyrazole is an important class of organic molecules and containing two nitrogen-atoms in five membered parent ring. In this synthesis approach features, newly substituted pyrazoles has been synthesized by condensation reactions between various substituted benzene-azo acetyl acetone (1a-1t) and substituted phenyl hydrazine with few drops of glacial acetic acid used as catalyst in alcoholic medium and substituted isoxazoles have been synthesized by the condensation reactions of subs. benzene-azo acetyl acetone (1a-1b) with hydroxyl amine hydrochloride and sodium acetate in the alcoholic medium. The structures of newly synthesized substituted pyrazoles and substituted isoxazoles were established on the basis of their spectral studies like IR, 1H NMR, Elemental Analysis, physical properties. The newly synthesized compounds were screened for their anti-bacterial activity against Staphylococcus aureus and Escherichia coli.
Keywords
Subs. benzene-azo acetyl acetone; Subs. Phenyl hydrazine; Pyrazoles; Isoxazoles; Antibacterial activity
Download this article as:| Copy the following to cite this article: Pareek A. K, Joseph P. E, Seth D. S. Synthesis, Characterization Of Some New 1- (2, 5 - Dichloro Phenyl Hydrazino)-3,5-Dimethyl- 4-(Substituted Phenyl Azo) Pyrazoles And 3,5-Dimethyl- 4-(Substituted Phenyl Benzene Azo) Isoxazoles As Anti-Bacterial Agents. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Pareek A. K, Joseph P. E, Seth D. S. Synthesis, Characterization Of Some New 1- (2, 5 - Dichloro Phenyl Hydrazino)-3,5-Dimethyl- 4-(Substituted Phenyl Azo) Pyrazoles And 3,5-Dimethyl- 4-(Substituted Phenyl Benzene Azo) Isoxazoles As Anti-Bacterial Agents. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1378 |
Introduction
The chemistry of pyrazoles and it’s derivatives are an important class of organic molecules has been extensively studied for last few decades. Many of the pyrazole derivatives have been found to possess biological activity1. Pyrazole derivatives have been reported to possesses as anti-cancer2, anti-diuretic3, anti-helmentic4, hypoglycaemic5, fungicidal6, anti-inflammatory7, anti-diabetic8, anti-microbial9 activities . Substituted pyrazoles have pronounced sedative action on the CNS10. Pyrazole derivative-es are known to be therapeutically useful compounds11 such as Celecoxib, diclofenac, non-steroidal anti-inflammatory (NSAID’s) and anti-pyretic drugs.
Isoxazoles consist a class of five membered ring containing Nitrogen and Oxygen with diverse applications12, isoxazoles are well known for their biological properties13, 3,5-dimethyl isoxazole is a potential hypoglycaemic agent14, isoxazole derivatives have been reported to possess as anti-bacterial15, anti-tubercular16, anti-viral17, anti-tumor18 activities. In continuation of our earlier work19. In the present communication we wish to report here the synthesis of some new pyrazoles and isoxazoles.
Experimental
Material and Methods
All chemicals are used in the synthesis were of analytical grade and obtained from Sigma-Aldrich Company. All the mentioned melting points were determined in open capillary tubes and are uncorrected. The purities of the newly synthesized compounds were checked on silica-gel-coated AI plates (E-Merck). IR spectra were recorded in Kbr-disc method on Perkin-Elmer spectrum RX-1 FT-IR spectrophotometer at ST. John’s College, Agra. 1H NMR spectra was measured on Advanced Bruker DRX-300, using solution in DMSO d6. Chemical shifts are given in δ (ppm) and protons signals are indicated as: s = singlet, d = doublet, t = triplet, m = multiplet. Elemental analysis was performed on Elementor Vario EL III.
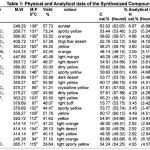 |
Table 1 |
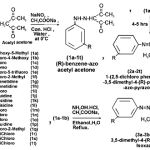 |
Scheme 1 |
General procedure for the synthesis of substituted phenyl benzene-azo acetyl acetone (1a-1t)
To the substituted aniline (0.025 mole) was diazotised by adding concentrated HCI (8ml) in distilled water (6ml), cooled the solution in an ice-bath at maintained temperature 0OC, after completing first step the cold aqueous solution of NaNO2 (0.025 mole) was added drop-wise in to the cooled diazotised solution, then this solution was added drop-wise in to the cooled maintained temperature 0OC solution of Sodium acetate (0.12 mole) and acetyl acetone (0.025 mole) in ethyl alcohol (25 ml), during stirring substituted benzene-azo acetyl acetone was separated out, filtered, washed with distilled water, recrystallized by hot ethanol.
General procedure for the synthesis of 1-(2, 5-dichloro phenyl hydrazino)-3,5 dimethyl- 4(substituted phenyl benzene-azo acetyl acetone) pyrazoles (2a-2t)
A mixture of (1a-1t ;0.001 mole) dissolved in absolute ethanol and (0.001 mole) of 2,5-dichloro phenyl hydrazine, then refluxed for 4-5 hours in the presence of 4 drops of glacial acetic acid. A coloured solid was separated after cooling the solution, filtered and purified by absolute ethanol 99% several times. It was identified to be 1-(2, 5-dichloro phenyl hydrazino) – 3, 5 dimethyl- 4(substituted phenyl benzene – azo acetyl acetone) pyrazoles.
General procedure for the synthesis of 4- (sub-stituted benzene – azo) -3,5-dimethyl isoxazole (3a-3b)
To (1a,1b ; 0.001 mole) dissolved in excess of ethanol (25 ml) was treated with aqueous solution of hydroxyl amine hydrochloride (0.01 mole) and Sodium acetate, then the mixture was refluxed for 4-hours on steam-bath, a coloured crystalline product obtained on cooling, filtered, recrystallized from ethanol 99%. It was identified to be 4 -(substituted benzene-azo)- 3,5 -dimethyl isoxazoles.
Elemental Analysis for C, H, N of Compound (2a) are as-58.62(58.57), 4.66(4.82), 14.39(14.72) and (2b)-52.76(52.73), 3.69(4.10), 13.67(13.85).
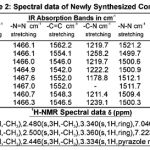 |
Table 2 |
Anti-bacterial Activity
The substituted pyrazoles (2a,2b,2e,2o,2j,2 q,3a,3b) were screened for antibacterial activity against one gram + ve Staphylococcus aureus and one gram – ve E.coli applying filter paper disc method20 at concentration of 25 µg ml-1 using Hi-Media Sterile disc SD-067 and Hi-Media Muller Hinton Agar Medium, using dimethyl formamide as a solvent, after 24 hours of incubation at 37O, the zone of inhibition were measured in mm.
The activity was compared with known antibiotic such as streptomycin and the results of antibacterial activity is listed in the Table-3.
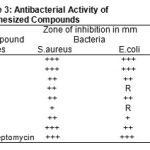 |
Table 3: Antibacterial Activity of Synthesized Compounds |
Key to Symbols: Resistance=R;slightly active=+(inhibition zone 6-9mm); morderately active = ++ (inhibition zone 9-12mm);highly active=+++(inhibition zone>12mm);
Most of the pyrazole and isoxazole showed significant antibacterial activity. The antibacterial activity is morderate to highly active of the compounds (2a,2b,3b) against gram positive S.aureus. The compounds 2a, 2b, showed morderate to highly anti-bacterial activity against gram -ve E.coli .
Results and Discussion
The IR Spectra of the newly synthesized compounds have been recorded in the frequency region 4000-500 Cm-1. The IR (Kbr-disc method) Spectral data and 1H NMR spectral data are recorded in the Table-2.
The IR Spectra of the compounds showed absorption bands in the range 3457.8-3414.5 cm-1 showed stretching vibrations of -NH, while absorption in the range 1467.6-1464.9 cm-1 indicates the pyrazole ring because of -N=N stretching vibrations, absorption bands in the range 1562.2-1542.0 shows the presence of -C=C stretching vibrations, absorption in the range 1258.2-1178.8 cm-1 indicates the -C-N stretching vibrations, stretching vibrations of -N-N in the range 1521.2-1499.7 cm-1, stretching vibrations in the range 1426.1-1420.8 cm-1 indicates the -CH3, stretching vibrations in the range 668.5-668.0 cm-1 reveals the mono substitution.
Thus the above observations are lent support to the assigned structure of compounds 2a-2f and other compounds 2g-2t.
IR spectrum of (3a,3b) shows absorption at 3451.6 cm-1, 3450.0 cm-1 indicates -NH stretching,absorptions at 1460.9 cm-1,1460.3 cm-1 reveals -N=N stretching vibrations, while absorption at 1548.3 cm-1 ,1546.5 cm-1 show aromatic -C=C, absorption at 1211.4 cm-1, 1239.1 cm-1 indicates the presence of -CN, absorption at 1509.4 cm-1 ,1500.3 cm-1 indicates the presence of -N-N, absorption at 1414.7 cm-1, 1414.2 cm-1 indicates the presence of -CH3,stretching vibrations at 668.5 cm-1, 668.4 cm-1 indicating the mono substitution .
The above observations are sufficient to support the assigned structure of the compound (3a,3b). The 1H NMR spectra showed singlet at δ 2.306, 2.428(CH3), 2.480,2.500(-CH3), 3.340,3.360(hydrazone ring), 7.046, 7.223(-C=O), these observations confirming the structures of compounds 1a,1b. The 1H NMR spectra showed singlet at δ 2.417(-CH3), 2.446(-CH3), 3.334(-pyrazole ring), 7.778(-C=O), these results are confirming the structure of the compound 2b and other compounds 2a, 2c-2t.
Acknowledgement
We are thankful to Central Drug Research Institute (CDRI), Lucknow for Spectral analysis (1H NMR) and Elemental analysis and Head of the Department of Botany ,R.B.S.College, Agra for Antibacterial Screening.
References
- G.G.Diana, P.M.Carabateas, G.L.William,I.Panicle and B.A.Stainberg,J.Med.Chem.,24,431 (1981)
- W.Wilson and N.Bottiglieri, Cancer Chemotheraphy, 21, 137(1962)
- H.G.Garg; J.Med.Chem.,15, 446(1972)
- H.G.Garg, N.Kaur; J.Med.Chem.,15, 554,(1972)
- H.G.Garg, V.Arora; J. Pharm.Sci., 61, 130(1972)
- H.Z.Khali,S.A.Vani; J.Indian Chem.Soc.,58,168 (1981).
- N.P.Shetgiri,A.D.Chitre, S.V.Kokitkar, S.M.Gh ate, S.S.Patil and R.C.Kelaskar, Indian J. Chem., 45b, 1308-1311(2006)
- F.S.G.Soliman and R.M.Shafik(Fac. Pharm.,Uni.Alexandria,Egypt)Pharmazie,30(7),436-39 (1975); C.A., 83, 193164b(1975)
- A.K.Mittal and O.P.Singhal, J. Indian Chem.Soc.; Vol.LVIII, 1089-90(1981)
- S.Salvi, P.T.Perumal, Indian J. Chem.Soc., 41B, 1887-1893(2002)
- A.Barco, S.Beneti, G.P.Pollini, D.Somani, Synthesis, 857(1987)
- D.H.Vyas, S.D.Tala, M.F.Dhaduk, J.D.Akbari and S.Joshi, J.Indian Chem. Soc., 84, 1140-1144(2007)
- W.E.Dulin and G.C.Geiristen, Proc.Soc.Eypte , Biol.Med., 113, 683(1953)
- T.Okuda. J.Kitamura and K.A.Azika.Proc.Gifu. Coll.Pharm., 5, 2083(1955)
- C.Caradonna and M.L.Stein,Pharmmaco,Edn. Sci., 15, 674(1960)
- N.Stelger, U.S.Pat.; 2, 555, 644(to Hoffman La RocheBosle); Chem.Abstr.; 45, 10259(1951)
- R.M.Kumbhare and Ingale; Asian J.Chem., 12 (4), 1317(2000)
- M.Scobie and M.D.Theadojill¸J.Org.Chem.,59, 7008(1994)
- Alok K.Pareek, P.E.Joseph and Daya S.Seth Orient.J.Chem.Vol. 26(1), 229-232(2010)
- C.A.Barry, J.L.Joyce, P.A.Adams, J.E.Bemer, Am.J.Clin.Pathol. , 59, 693(1973)







