A. Amala Lourthuraj1, M. Masilamani Selvam1 and Bharathi Ravikrishnan2
1Department of Biotechnology, Sathyabhama University, Chennai, India. 2Department of Biotechnology, Hindustan College of Arts and Science. Chennai, India. Corresponding Author E-mail: a.amalraaj@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1105
Abstract
Currently in India the population of people suffering from lung cancer is increasing at an alarming rate. Chemotherapy is widely used for the management of lung cancer; however it has various side effects. Hence, there is a need to find newer therapeutic agents with fewer side effects. Plant derived substances are generally considered as the most preferred alternative medicines. Thus, in this study, we have tested the antiproliferative property of Cleistanthin-A, a chemical compound extracted from dried leaves of Cleistanthus collinus, against the A549 lung cancer cell lines. The antiproliferative assays used are dye exclusion assay, MTT assay and Comet assay.
Keywords
Cleistanthin-A; anti-proliferative assay; A549 cell lines
Download this article as:| Copy the following to cite this article: Lourthuraj A. A, Selvam M. M, Ravikrishnan B. Analyses of Antiproliferative Property of Cleistanthin-A Against A549 Cell Line. Biomed Pharmacol J 2017;10(1) |
| Copy the following to cite this URL: Lourthuraj A. A, Selvam M. M, Ravikrishnan B. Analyses of Antiproliferative Property of Cleistanthin-A Against A549 Cell Line. Biomed Pharmacol J 2017;10(1). Available from: http://biomedpharmajournal.org/?p=13556 |
Introduction
Changing lifestyles, urbanization, globalization and progressive control of communicable diseases have led to emergence of cancer and other non communicable diseases as an important health problem in India. The environmental risk factors for lung cancer are carcinogen and co-carcinogen which are present in tobacco and its related products (1). Cigarettes are the most important tobacco product worldwide (http://tobaccopedia.org). It is estimated that cancer incidence would increase to 1,869,983 (1.87 million) by the year 2026. The International agency for research on cancer called as Globocan project has predicted that in India patients with cancer by 2035 will be nearly 1.7 billion (2). In India, approximately 63,000 new lung cancer cases are reported each year (3). Apart from tobacco related products, other causative agents for lung cancer are radon gas, asbestos, silica, and diesel exhaust, excess exposure to air pollution, chronic lung infection like tuberculosis (www. Cancer research uk.org /about –cancer /type /lung-cancer/ about/lung-cancer-risks-and-causes). Tobacco smokers above age 50 years are at high risk for lung cancer (4). Lung cancer is also a hereditary disease (5). In non-smokers, the etiology of lung cancer is due to the environmental, hormonal, genetic and viral implications (6). Reactive oxygen species (ROS) are recognized to play a dual role in both regulation of physiological reactions and also leading to oxidative stress which generates a negative bioniche for the generation of chronic diseases like cancer (7). Antioxidants like vitamins- A, C and E were thought to help reduce the risk of lung cancer, but evidence for this is not clear (www.cancerresearchuk.org/about-cancer/type/lung-cancer/about/lung-cancer-risks-and-causes).
A549 cell line is alveolar basal epithelial cells. A549 cell lines are widely used as an in vitro model for a type II pulmonary epithelial cell model for drug metabolism and as a transfection host (8-9). The mRNA of nicotinic acetylcholine receptor α7 is expressed in all the human cancer cell lines (10). nAChR genes are expressed in both in neuronal cells and also in other non-neuronal tissue cells like skin, pancreas and lung. Through this it is evident that nAChRs can play vital role in other biological processes in addition to synaptic transmission (11-12). Nicotine result in influx of calcium ions into lung cancer cells as the result of it’s binding to α7nAChR and thus causes membrane depolarization which in turns activates the MAPK pathway, which may result in the elevated expression of B-cell lymphoma-2 protein, thus down-regulating apoptosis of such cells (13).
In the present work, the Cleistanthin-A fraction extracted from the dried leaf of poisonous plant Cleistanthus collinus (Oduvanthalai in Tamil) (unpublished), was used as an anti-proliferative agent, to inhibit the proliferation of A549 cell line. It is interesting that this toxin also has curative properties. Extract of various plant parts yielded a multitude of compounds of which the glycosides, arylnaphthalene lignan lactones are toxic (14). These lignan lactones include Cleistanthin-A and B (15), collinusin and diphyllin. Mortality is attributed to cardiac arrhythmias1, 4 acute renal failure, shock and respiratory failure in humans.
Methodology
Compound Isolation
Cleistanthin-A, the active principle of the plant Cleistanthus collinus, was isolated using a novel method established by the department of Physiology, Christian Medical College, Vellore (Unpublished). This isolated compound was characterized initially by thin layer chromatography profiling followed by UV & FT-IR and the antioxidant property by DPPH activity was analysed (16).
Sample used
1mg of the Cleistanthin-A was suspended in 1ml of DMSO and 1:3 dilution was prepared for the MTT assay (17).
Maintenance of A549 Cell line
A549 cell lines were obtained from National centre for cell sciences, Pune (NCCS). The cells were maintained in MEM supplemented with 3.75 gms sodium hydrogen carbonate, 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere of 5% CO2 at 37 °C (18). After the monolayer has established after four days of incubation, the cells were subjected to trypsinisation (0.25% trypsin and 1mM EDTA in Dulbecco’s Phosphate Buffered saline (DPBS), without calcium chloride and magnesium chloride, ± 7.2) and after three minutes of incubation at 37°C in 5% CO2 the trypsinisation was stopped by the addition of 1 ml of FBS. The cells were then subjected to washing process with DPBS (19).
Trypan Blue Dye Exclusion test
The cells were rinsed with DPBS, to remove trypsin if any. Then the cells were suspended in sterile DPBS. 0.4% of trypan blue dye was added to the cell suspension. 20μl of cell suspension was loaded onto a hemocytometer and cell count was performed. Viable cells would have excluded trypan blue and the total viable cells were recorded. MTT assay was further performed for the confirmation of the viability (19).
Cell Viability Assay
The anticancer activity of Cleistanthin-A on Lung cancer cell line (A549) was determined by the MTT assay (20). Cells (1 × 105/well) were plated in 0.2 ml of medium/well in 96-well plates and cultured in a 5 % CO2 incubator at 37°C for 72 hours. Various concentrations of Cleistanthin-A in 0.1% DMSO were then added and the cells cultured for a further 24hrs. The supernatant was removed and 20μl/well (5mg/ml) of 0.5% 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl–tetrazolium bromide (MTT) in phosphate- buffered saline solution was added to all the wells. After 4hrs incubation, 1ml of Dimethyl Sulfoxide (DMSO) was added. The contents of the well were transferred to a fresh sterile 1.5 ml microfuge and were subjected to centrifugation at 18°C for 1500 rpm, to remove the cell debris. Viable cells were determined by the absorbance at 540nm. Measurements were performed and the concentration required for a 50% inhibition of viability (IC50) was determined graphically. The effect of the samples on the proliferation of A549 cells was expressed as the % cell viability, using the following formula:
% cell viability = A540 of treated cells / A540 of control cells × 100
Comet Assay
Comet assay was performed (21-22). About 1×105 cell of A549 were seeded in 24-well tissue culture plates and after 24 hours of incubation at 37°C and in 5% CO2 humified atmosphere, various concentration of Cleistanthin-A suspended in DMSO cells and the untreated control cells were analysed for the comet tail length, which confirms the DNA degradation patterns.
Result
After the A549 cells were cultured in MEM medium, for all the following analysis the cell concentration of 1×105 / well of cells were used. The trypan blue dye exclusion test, demonstrated 80% cell viability. Cleistanthin-A reduced the number of viable cells which is evident through the MTT assay (Figure.1). At 100 μg/ml concentration of Cleistanthin-A, all cells were killed. IC50 concentration of the Cleistanthin-A suspended in DMSO was determined graphically as 6.25 μg/ml for the, lung cancer line (Table.1 and Graph.1). All the experiments were performed in triplicates and the graph was generated by using Microsoft excel 2007 edition.
 |
Figure.1: MTT ASSAY |
Table 1: MTT assay
| S.No. | Concentration, µg/ml | Absorbance, 540nm | % cell Viability |
| 1 | 100 | 0.01 | 0.9 ± 0.002 |
| 2 | 50 | 0.05 | 4.8± 0.004 |
| 3 | 25 | 0.11 | 10.5± 0.002 |
| 4 | 12.5 | 0.20 | 19.2± 0.008 |
| 5 | 6.25 | 0.43 | 41.3± 0.003 |
| 6 | 3.12 | 0.62 | 59.6± 0.067 |
| 7 | 1.56 | 0.74 | 71.1± 0.004 |
| 8 | Control cells | 1.04 | 100 ± 0.002 |
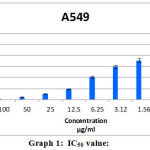 |
Graph 1: IC50 value |
Discussion
Currently even though variety of chemotherapeutics available for lung cancer, there is need for newer drugs with less toxicity (23). Nicotine can induce the activation of NF-κB through the MAP kinase and PI3K/AKT signaling pathways, which promote survival, proliferation, and angiogenesis of endothelial cells (24). The current chemotherapy either in combined form or in individual forms are not successful (25). Hence this current investigation could pave way for developing suitable therapy for lung cancer. Thus the A549 cell line was chosen as the suitable model to study the effectiveness of Cleistanthin-A as an antiproliferative compound. The compound Cleistanthin-A has a good antioxidant property. These could be the suitable reasons for the compound Cleistanthin-A contributing to the antiproliferative property against the A549 cell line. Further in silico studies of Cleistanthin-A binding effectively to nAChRs receptors was also studied by patch dock (26). Further studies must elucidated in relation to the analyses of pharmacokinetics and pharmacodynamics to confirm the role of Cleistanthin-A as a good anticancer drug for lung cancer in case of both non-smokers and smokers.
Conclusion
In this present study it is evident that Cleistanthin – A can reduce viability of A549 cell line, a lung cancerous cell line model.
Acknowledgement
The authors would take the privilege to thank Physiology Department of Christian Medical College, Vellore and Royal Lab, Velachery, Chennai for their technical suppor
References
- Beasley, M.B, Brambilla E., Travis W.D. (2005). The 2004 World Health Organization classification of lung tumors. Semin Roentgenol ;40:90–97.
- Mohandas, M.K., David, G.T., Rajendra, A. B., Goura, K. R., Shanta, V., Pramesh, C.S. Raghunadharao, D., Paul, S., Bibhuti, B.B., Ashok, K., Sanjay, K., Shaleen, K., Jennifer, L. G., Moni, A.K., Hemant, M., Suresh, C.S., Shilin S., Lokesh, V., Raju, T.C., Jeremy, L.P., Kenipakapatnam, S.R., Kailash, .S.S., Arnie, D.P., Richard,S., (2014). The growing burden of cancer in India: epidemiology and social context. Oncology, The Lancet; 14: 70115-9.
- Ganesh B., Sushama S., Monika S and Suvarna P. (2011). A Case-control Study of Risk Factors for Lung Cancer in Mumbai, India. Asian Pac J. Cancer Prev.; 12:357-62.
- Philip C.T., Allen, T.C., Beas, S., Borczuk, A.C. and Kerr, K.M. (2012). Etiology of Lung Cancer. Molecular pathology. ISBN: 978-1-4614-3196-1: 5-7.
- Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer; 136 (5) :E359–86.
- Noronha, V., Dikshit, R., Raut, N., Joshi, A., Pramesh, C.S., George, K., Agarwal, J.P., Munish, A and Prabhash, K. (2012). Epidemiology of lung cancer in India: Focus on the differences between non-smokers and smokers: A single-centre experience. Indian Journal of cancer; 49 (1): 74-81.
- Valko, M., Rhodes, C.J., Moncol, J., Izakovic, M and Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact; 160: 1-40.
- Foster, K.A, Oster, C.G., Mayer, M.M., Avery, M.L., Audus, K.L.(1998). Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 243(2): 359 -366.
- Zhang Z., Zhang L., Yin Z.Y., Fan. X.L., Hu B., Wang L.Q. and Zhang D. (2014). miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int.J.Clin.Exp.Pathol. 7(10): 7236-7241.
- Howard Plummer, K., Madhu Dhar and Hildegard Schuller, M. (2005). Expression of the α7 nicotinic acetylcholine receptor in human lung cells. Respiratory Research; 6:29.
- Grozio, A., Paleari, L., Catassi, A. (2008). Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a promising prospective in anticancer drug development. Int. J. Cancer; 122:1911–1915.
- Bierut, L.J. (2009). Nicotine dependence and genetic variation in the nicotinic receptors. Drug Alcohol Depend ; 104 (1) :S64–S69.
- Heusch, W.L. and Maneckjee, R. (1998). Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis; 19: 551–556.
- Thomas, K., Dayal, A.K., Ganesh, A., Seshadri, M.S. and Cherian, A.M. (1991). Metabolic and cardiac effects of Cleistanthus collinus poisoning. J. Assoc. Physicians India; 39:312–4.
- Eswarappa, S., Chakraborty, A.R, Palatty, B.U. and Vasnaik, M. (2003). Cleistanthus collinus poisoning: Case reports and review of literature. J Toxicol Clin Toxicol.;41:369–72.
- A. Amala Lourthuraj, M. Masilamani Selvam, Bharathi Ravikrishnan, Murugan R. Therapeutic properties of a poisonous plant Cleistanthus collinus. Research J. Pharm. and Tech. 9(4): April 2016.
- Rajesh, M.P. and Sahil, K.P. (2011). Cytotoxic activity of methanolic extract of Artocarpus heterophyllus against A549, Hela and MCF-7 cell lines. J.of applied pharmaceutical science; 01(07): 167-171.
- Giard, D.J., Aaronson, S.A., Todaro, G.J., Arnstein, P., Kersey, J.H., Dosik, H. and Parks, W.P. (1973) : In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 51(5): 1417-1423.
- Ian Freshney, R. (2010). Culture of animals cells, A manual of basic technique and specialized applications. 6thEdition.Wiley Blackwell.
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. of lmmunological Methods, 65 (55):55-63.
- Tice, R.R, Agurel, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., Miyamae, Y., Rojas, E., Ryu, J.C. and Sasaki, Y.F. (2000). Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing, Environmental and Molecular Mutagenesis; 35: 206-221.
- Muthukumari, D., Padma, P.R. and Sumathi, S. (2013). In Vitro Analysis of Anethole as an Anticancerous Agent for Triple Negative Breast Cancer. Int. J. Pharm. Sci. Rev. Res., 23(2): 314-318.
- MER O’Brien, A., Borthwick , A., Rigg, A., Leary, L., Assersohn , K. L., Tan, S., Milan, S., Tait, D. and Smith, I.E. (2006). Mortality within 30 days of chemotherapy: a clinical governance benchmarking issue for oncology patients. British Journal of Cancer; 95: 1632 – 1636.
- Yu, J., Huang, N.F. and Wilson, K.D. (2009). “NAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis,” PLoS ONE, 4(9)- doi: 10.1371/journal.pone.0007040.
- Behera, D. (2007). New Approach to the Treatment of Lung Cancer: The Molecular Targeted Therapy. Indian J. Chest Dis. Allied Sci. 49: 149-158.
- Amala Lourthuraj, A., Masilamani Selvam, M., Bharathi Ravikrishnan and Vinoth, M. (2015). Bioprospective of Cleistanthin-A in a bioinformatics approach. 11th International Conference on Science and engineering and Technology: 149-153. ISBN 978-93-85477-73-7.








