Ali Hattem Bayati1, Yasmeen J. Al-Bayaa2, Sevan Najem Alwan3 and Ibrahim Isam Al-Karkhi4
1Department, Technical College of Health, Sulaimani Polytechnic University, Sulaimani city, Kurdistan Region/Iraq.
2,3Department of Microbiology , Baghdad Medical College, Baghdad, Iraq.
4Memorial University of Newfoundland, NL, Canada.
Corresponding Author E-mail: dr.alkarkhi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1101
Abstract
Among many viral causes of miscarriage, maternal infections caused by Cytomegalovirus and Epstein-Barr virus infections are important causes. This study aimed to detect the possible occurrence of Cytomegalovirus and Epstein-Barr virus infections in placental tissues from patients with spontaneous abortion using immunohistochemistry and in situ hybridization techniques. Immuno-histochemistry technique and chromogenic in situ hybridization assay was used to detect placental infection with Cytomegalovirus and Epstein-Barr virus in 40 women with spontaneous miscarriage and in 40 healthy delivery n Baghdad/Iraq. Equal detection rates of Epstein-Barr virus in placental tissues by either CISH or IHC were (22.5%), yet the validity results of Epstein-Barr virus - VCA by IHC as compared to Epstein-Barr virus - EBER by CISH have displayed a sensitivity of 44.4% and 83.9%, respectively. The detection rates of Cytomegalovirus -DNA by CISH and Cytomegalovirus -protein by IHC were (30%), (37.5 %), respectively. The results of Cytomegalovirus -DNA -ISH as compared to this Cytomegalovirus - IHC-protein had revealed a sensitivity and specificity of 41.7% and 64.3%, respectively. Cytomegalovirus and Epstein-Barr virus are important causes of placental infections among miscarriage females in Baghdad, and Cytomegalovirus might be detected in placenta of normal delivery. Although CISH technique considered as the gold standard method for detecting of latent Epstein-Barr virus and /or Cytomegalovirus infection were IHC has showed a compatibility to that technique and might reach rates of high sensitivity and specificity similar to it.
Keywords
Cytomegalovirus; Epstein-Barr virus; immunohistochemistry; chromogenic in situ hybridization; miscarriage; pregnancy; placenta
Download this article as:| Copy the following to cite this article: Bayati A. H, Al-Bayaa Y. S, Alwan S. N, Al-Karkhi I. I. Detection of Cytomegalovirus and Epstein Barr Virus in Placental Tissues of Aborted Women. Biomed Pharmacol J 2017;10(1) |
| Copy the following to cite this URL: Bayati A. H, Al-Bayaa Y. S, Alwan S. N, Al-Karkhi I. I. Detection of Cytomegalovirus and Epstein Barr Virus in Placental Tissues of Aborted Women. Biomed Pharmacol J 2017;10(1) Available from: http://biomedpharmajournal.org/?p=13545 |
Introduction
The abortions reasons in several circumstances yet are mysterious, nevertheless the bacterial toxicities signify a main reason in abortion, where germs seems to be the utmost elaborate pathogens (Khameneh et.al., 2014) and (Oliver and Overton ,2014).
Between numerous germs, Humanoid Herpes germ toxicities of placenta which could be hurtful in pregnant foremost to complaints in the growth of fatality, early delivery, failure, or main irregularities, besides it could yield to a prolonged or frequent parental infection. In specific, CMV through pregnancy could spread the placenta by viremia, subsequent to main and recurring toxicity, or by rising way as of the cervix, regularly subsequent recurrence. The germ with a smallest mid herpes viruses, Epstein-Barr virus which usually related with irregular abortions (Avgil and Ornoy, 2006) and (Di Stefano et.al., 2008).
The goal of the current research is to investigate the differences in the presence of two herpetic virus-related toxicities inside placental soft tissue as of caseswhose spontaneously abortion through determination of infections with CMV, and EBV involving the placenta as a possible causes for subsequent abortion by using the following techniques: Immunohistochemistry and In situ hybridization; as well as comparing the two techniques for detection of CMV, and EBV.
Method
This retrospective study made the use of paraffin contain placental soft tissue that composed from histo-pathological annals of Training Labs form the hospital of AL-Yarmouk in Baghdad / Iraq and belongs to forty (40) feminine cases with miscarriages as patient’s collection; the range of the ages were (19-43 years), and 40 placental soft tissue of normal delivery as a control group. Depiction Rabbit and Mouse with certain HRP \DAB inspection IHC Kit ab80436 (2013) Abcam was used for inspection of CMV –protein and Epstein Barr – viral capsid antigen (EBV –VCA) specific primary antibodies . CISH Implementation AP-NBT kit for detection of EBV (EBER – RNA) and CMV (DNA) for chromogenic in situ hybridization (CISH) using biotin – labeled Zyto Fast CISH probe was purchased from ZytoFast/Germany Cat. Numbers (T-1070-40 – 2011). Numerical investigation: Investigation of statistics was achieved by means of the obtainable numerical set of (SPSS-22).
Results
For IHC technique, the expression of EBV –VCA IHC signs were identified as a brown color staining localized at nuclear (Figure1, A). The placental soft tissue tasters of aborted women presented 22.5% (9 cases from 40), whereas not any of well control placental soft tissue displayed EBV – VCA antigen expression. The maximum ratio of EBV-IHC shows a modest sign 66. 7 % (6 cases from 9) as depicted in Table 1.
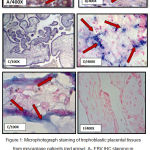 |
Figure 1: Microphotograph staining of trophoblastic placental tissues from miscarriage patients (red arrow): A- EBV IHC staining in cell nucleus intensity. B- IHC staining for CMV at cell nucleus. C- Negative IHC staining for EBV. D- EBV-ERER CISH positive signals in the cell nucleus of trophoblastic placental tissues showed score 2 and moderate intensity. E-CMV -DNA staining CISH of trophoblastic placental tissues in the cell nucleus score 1 and weak intensity. F- Negative CISH staining for EBV. |
Table 1: Immuno-histochemical scoring & signal intensity data of EBV –VCA
|
Groups
|
Miscarriage
Set |
Control
Set |
P value | |||
| Number | % | Number | % | |||
| EBV IHC Positively Score | Negatively | 31 | 77.50 | 40.00 | 100.00 | |
| Positively | 9 | 22.50 | ||||
| (Score I) | 7 | 77.80 | ||||
| (Score II) | 2 | 22.20 | ||||
| (Score III) | ||||||
| EBV IHC Positively Intensity
|
(Weakly / I) | 3 | 33.30 | |||
| (Moderately / II) | 6 | 66.70 | ||||
| (Strongly / III) | ||||||
The general appearance of CMV protein at nuclear localization (Figure1, B) was distinguished in 37.5% of the placental soft tissue of failure group and in 5% of healthy placental soft tissue. A great percent (around 60%) among placental soft tissue in the failure group had feeble score (score I). The uppermost positive CMV-IHC reactions in those with miscarriage group were showed strong signal intensity 66.7% .Statistically, noteworthy changes (p <0.05) was distinguished associating with the infection degree among miscarriage and control group (Table 2).
Table 2: CMV-protein of IHC in placental soft tissue
|
Groups
|
Miscarriage
Set |
Control
Group |
P value | |||
| No. | % | Number | % | |||
| CMV IHC Positively Score | Negatively | 25.00 | 62.50 | 38.00 | 95.00 | 1x 10-4* |
| Positively | 15.00 | 37.50 | 2.00 | 5.00 | ||
| (Score I) | 9.00 | 60.00 | 2.00 | 100 | ||
| (Score II) | 1.00 | 6.70 | ||||
| (Score III) | 5.00 | 33.30 | ||||
| CMVIHC Positively Intensity | (Weakly / I) | 1.00 | 6.70 | 2.00 | 100.00 | |
| (Moderately / II) | 4.00 | 26.70 | ||||
| (Strongly / III) | 10.00 | 66.70 | ||||
*Very clear differences among the ratios by (Pearson Chi-square test at 0.05 level)
The chromo-genic are in situ hybridization (CISH) system, the whole percent of positive CISH of EBERS between placental soft tissue in the miscarriage group was 22.5%, while none of the 40 control placental tissues have revealed positive CISH- signals for this EBV marker (figure 1, D). The scoring of EBER RNA- CISH reactions was in its highest percentage 66.7% of all positive cases in the low score (score I), while the their signal intensity of the color development where the percentage of EBER- RNA –CISH reactions that showed weak, moderate, and strong intensities were 4(44.5%), 3(33.3%), and 2(22.2%) respectively (Table 3).
Table 3: Distribution of scoring and intensity of CISH actions for EBER RNA- in placental tissues of miscarriage and control group.
|
Groups
|
Miscarriage
Group |
Control
Group |
P value | ||||
| No | % | No | % | ||||
| EBV CISH Positively Score | Negatively | 31.00 | 77.50 | 40.0 | 100.00 | ||
| Positively | 9.00 | 22.50 | |||||
| (Score I) | 6 | 66.70 | |||||
| (Score II) | 3 | 33.30 | |||||
| (Score III) | |||||||
| EBV CISH Positively Intensity
|
(Weakly / I) | 4 | 44.4 | ||||
| (Moderately / II) | 3 | 33.3 | |||||
| (Strong / III) | 2 | 22.2 | |||||
CMV DNA was noticed in (30%) from the studied placental soft tissue in the miscarriage set, whereas placental tissues of healthy delivered women have revealed in (15%). Regarding the signal scoring, the most frequently detected CMV – positive placental tissue in the miscarriage group were observed to have the lower score (score I), (50%), 58.3 % revealed weak sign strength ,numerically, no difference observed (p >0.05) as depicted in (Table 4).
Table 4: Distribution of HCMV DNA CISH result of the examined placental tissues of miscarriage and their counterpart control tissues
| CMV CISH signal Score & Signal intensity | Miscarriage Group | Control Group | P value | |||
| Number | % | Number | % | |||
| CMV CISH Positive Score | Negative | 28.00 | 70.00 | 34.00 | 85.00 | 0.108 |
| Positive | 12.00 | 30.00 | 6.00 | 15.00 | ||
|
CMV CISH Positive Intensity
|
Score I | 6.00 | 50.00 | 5.00 | 83.30 | 0.308 |
| Score II | 3.00 | 25.00 | 1.00 | 16.70 | ||
| Score III | 3.00 | 25.00 | ||||
| weak / I | 7.00 | 58.30 | 6.00 | 100.00 | ||
| Moderate /II | 5.00 | 41.70 | ||||
| strong / III | ||||||
The Validity of CISH and IHC techniques for diagnosing placental tissues of miscarriage patient infected with EBV showed that of the total 40 placental tissues in the miscarriage group, nine have been identified to express CISH reaction for EBERs and IHC reaction VCA. No numerically important differences between these results of the two detection methods (P<0.005). The statistical observed sensitivity and specificity were 44.4% and 83.9%, for CISH and IHC for detection of EBV in placental tissues of miscarriage patients respectively (Table 5), while the validity of CISH and IHC techniques in diagnosis CMV placental infections showed that the percentage of positive –reaction results of CMV –DNA -CISH in placental tissues of miscarriage patients was 30% while CMV-IHC protein expression in placental tissues of miscarriage group was 37.5% . The number of cells in serial sections that showed positive IHC was slightly greater than those have showed CISH positive reaction. Labeling results of both in situ hybridization and immunohistochemistry were showed in five cases. Statistically the observed sensitivity and specificity were 41.7% and 64.3%, for CISH and IHC for detection of CMV in placental tissues of miscarriage patients, respectively (Table 6).
Table 5: Validity of EBV- CISH and EBV- IHC
|
EBV IHC Score |
Miscarriage Group | |||
| EBV CISH | ||||
| Negative (n=31) | Positive (n=9) | |||
| No | % | No | % | |
| Negative | 26.00 | 83.90 | 5.00 | 55.60 |
| Positive | 5.00 | 16.10 | 4.00 | 44.40 |
| P value | 0.073 | |||
Table 6: Validation of CMV for DNA CISH and for CMV protein IHC among the placental miscarriage tissues.
|
CMV IHC Score |
Miscarriage Group | |||
| CMV CISH Score | ||||
| Negative (n=28) | Positive
(n=12) |
|||
| No | % | No | % | |
| Negative | 18 | 64.30 | 7.00 | 58.30 |
| Positive | 10 | 35.70 | 5.00 | 41.70 |
| P value | 0.722 | |||
Discussion
EBV could be the reason of infection to placenta in pregnancy, with consequent complications fetus. Primary and secondary EBV infections may both occur during pregnancy. However, main infections with EBV seems trans placental diffusion were occasional, whereas secondary maternal EBV infections weren’t rare (Avgil and Ornog 2006).
Most of laboratory tests that were performed to diagnose EBV infections (including infections during pregnancy) use molecular techniques (like PCR) and serological tests for EBV antigens or antibodies specific to EBV by ELISA, immunofluorescent technique, or rapid tests (Christian et al., 2012). In this study, 9 out of 40 (22.5%) of the examined placental tissues were positive for EBV (EBER) by CISH technique. Also the appearances of EBV-VCA were distinguished at nuclear localization in 22.50% (9 out of 40). In the current study which is presented here, a partial measurable CISH method was used for scoring of bleach growth, according to the number of placental cells infected with EBV and the results showed that most of infections have of score I, and this might represents the comparative mild to reasonable EBV infections of placental tissue through pregnancy as well as these findings were supported by measurement of color intensity of positive cases were most of the examined slides were having mild signal intensity.
In the present study, the positive placental tissues of miscarriage group may indicate recently infected, or lytically reactivated EBV infection, however, the presence of EBER as detected by CISH, does not always correspond with the presence of VCA by IHC.
Immunohistochemistry was applied for detection VCA of EBV in placental tissue samples and it showed compatibility of those obtained by ISH and this might indicate a high compassion and specially the IHC-VCA test, since the EBER in situ hybridization for detecting of latent EBV infection is considered the gold standard method and as supported by the finding of Truong et. al., 2009. IHC has also been used where in this regard , Xiao and his worker, 2014 have showed also strong agreement for both techniques which were used for detection EBV in patients with lupus nephritis.
The present result are supported by Xuan-Hong (2011) who suggested that the possibility of the EBV virus to transmission from the uterus to the fetus , which were resulting in stillbirth , abortion , or congenital defects.
The current study provide a novel information about the histological localization of EBV nucleic acid in placental tissues using CISH, were the overall frequency of EBV infection is 22.5 % of the examined placentae, the present result is higher than those reported by other abroad studies such as that done by Gervasi and his colleagues (Gervasi et al.,2012 ) who found viral genomic sequences of EBV (0.1%) of their examined cases of the amniotic fluid ( in the mid trimester of pregnancy) by using quantitative real-time PCR for the presence of EBV-genome sequences. Recent studies done by Song and his Colleagues in 2015, used ISH for detection EBV in lympho-proliferative disorders, nasopharyngeal carcinoma and related malignancies with final color or fluorescence detection however, there is no reported data on detection of EBV by ISH during pregnancy and this signify a novelty for this study(Song, 2015 ).
Human cytomegalovirus is an significant etiological agent of intrauterine infection, which may lead to some thoughtful consequences in pregnant women such as miscarriage, stillbirth, cerebellar malformation and fetus development retardation, as depicted in Staar et., al 2012.
The results of this study was in line with a serological study done in Iraq to discover the association of TORCH infections in women with spontaneous abortions , CMV Ab prevalence of positive cytomegalovirus CMV(38.50%) in 2009 and , the lowest percentage were observed only (29.10%) of cases in 2010. This could suggest that the exposure to CMV infection was declined over this period in Iraq. (Majeed, 2011)
The study that was achieved in Baghdad by (Maysara et., al 2012) to evaluate the prevalence of seropositivity of specific IgM antibody for CMV by ELISA, CMV specific IgM antibody was detected in 15.7% of the 108 women with history of abortion.
In Australia, (Jenna et., al 2015) conclude to the result that the total CMV DNA was distinguished in 5.0 % of placenta soft tissue of miscarriage women along with the infections were confirmed by using the immune-histochemical assay and viral proteins.
Additional seroepidemiological research was made in Havana which was made by (Aimée et., al 2015) at maternity hospital by means of marketable ELISA kits has shown that the prevalence of active cytomegalovirus infection was detected in 16.70 %,.
Our results disagree using a previous outcomes done by Sharief,( 2005) , who showed an absolute positive results of aborted women with a primary abortion i.e reveled highest percentage (100% ) which was considered to carry a high titer of IgG. In developed countries such as United States it was stated that HCMV infection at rate of 80% was observed at age 35-40 years old women who have anti- HCMV IgG in comparison to 50% -80% HCMV infection in the younger age women (Gold, and Nankervis, 2007). The hematogenous way of CMV diffusion in the placenta can clarify the principal infection to this microbe in the variable villi.
The current study revealed that sensitivity of IHC is slightly higher than that of ISH for histological detection of CMV, this might be due to CMV protein over expression (which was studie here) can be detected by IHC but not by ISH, similar result were also obtained by LU DY and his colleagues (2009) found similar findings that the result of IHC is higher than those of ISH; in our opinion the CISH is more difficult to done and this might slightly affect the sensitivity of the procedure.
Yiska et., al (2011) did an Immunohistochemical analysis to infected units to parental decidua exposed the appearance of CMV immediate-early and pp65 early-late virus-related genetic factor and gB as well which stated late later infection. The control tissues were found negative by immunohistochemical staining. These findings indicate that HCMV, in the infected placental tissues undergoes a full replication cycle.
While this research dominant proportion of control group (6 from 40 using CISH) and (2 from 40 using IHC), this reflect to CMV positivity in these placental soft tissues in controller groups may reflect the need for following the studies values of the infections of such pregnancies both on toddlers and mothers subsequently later the deliveries cause the abortion consequences of placental CMV infection thru pregnancy and postnatal consequence should be well thought-out as well.
The present research follow the inclusion patients with abortions, and omitted the other results of infected pregnancy like fetal inherited anomalies and illnesses, it represent a restriction to this study which was established by recording as well as the difficult understanding of CMV infection in the control placental tissue group.
In study in Iraq , Alwan and Sera (2011) have indicated that CMV infection rate was (46.6%),These results were higher than our results , this different in the CMV prevalence rate may be due to different in the sample size included in the study group as well as may be due to substantial diffusion of infection in local population , perhaps that CMV is endemic in Iraq during that period , or may be CMV infections are un common in a particular region .
The current results obtained from both techniques for EBV have revealed that among Forty placental tissues from miscarriage patients , EBER- CISH and VCA-IHC have been found in 22.5% . While the detection rates of EBV-CISH and EBV-IHC in placental tissue sections were equivalent yet the validity results EBER by ISH where compared to VCA by IHC had displayed a sensitivity and specificity of 44.40 % and 83.90 %, respectively,
Also in this study the evaluation of the efficacy of these two procedures but for detection the presence of CMV infection in placental tissues from miscarriage patients. Both CISH and IHC for diagnosing nuclear inclusions of this virus in placental tissues have showed that , CMV-DNA- CISH and CMV-protein -IHC were found in 30% , 37.5 % respectivly. The comparism of the results of both technique revealed a sensitivity and specificity of 41.7% and 64.3%, respectively. The numbers of infected cells scored were slightly higher with IHC than with in CISH technique. These results show that the use of both in CISH and IHC for EBERs and the EBV- VCA as well as CMV –DNA and CMV proteins are both specific, as well as sensitive methods.
The current result of this study are matching with Niedobitek et al.,(1988) result . They used ISH, IHC, and morphological analysis of tissue from patients with AIDS who have widespread CMV infections. They showed that the evaluation of the results in ISH and IHC were significantly extra sensitive than the morphological analysis. It was further shown that IHC has shows a higher CMV infection in cells than in CISH. They concluded that IHC as technique appeared to be more suitable to be used.
Several authors have denoted that ISH was considered as a method of optimal to detect EBERs –EBV presence inside tissues section and this is because of the huge data of duplicates of EBERs present in infected cell where these works have showed Positive staining in the nuclei of the EBV-infected cells (Weiss and Chen , 2013; Hänel et.al., 2001; Margaret and Weihua , 2008 ).
In addition our data were match with the fact that ISH was considered as the gold standard for precisely identifing the sites of expression of EBV as it and was highly sensitive and specific; however, some authors denoted that ISH is expensive (Lerner et al. ,1981). The results of the current study showed that the two detection techniques had strong agreement, which in turn indicated that the results of both were reliable.
In conclusion CMV and EBV are important causes of placental infections among miscarriage females in Baghdad, and CMV might be detected in placenta of normal delivery. Although CISH technique considered as the gold standard method for detecting of latent EBV and /or CMV infection were IHC has showed a compatibility to that technique and might reach rates of high sensitivity and specificity similar to it.
References
- Aimée Festary, Vivian Kourí, Consuelo. Correa, Denis Verdasquera, (2015) Cytomegalovirus and Herpes Simplex Infections in Mothers and Newborns in a Havana Maternity Hospital Tania Roig MD MS PhD, Martha P. International Journals Vol 17, No 1 Peer Reviewed 29.
- Alwan, A.Sera . (2011) interleukin-10 and interlukin-12 level among Iraqi aborted women infected with human cytomegalovirus .Higher Diploma thesis.Health and Medical Tachnical College .Foundation of Technical Education.
- Avgil M. ; Ornoy A.( 2006). Herpes simplex virus and Epstein-Barr virus infections in pregnancy: consequences of neonatal or intrauterine infection. Reprod Toxicol. 21(4):436-45.
- Aziza Khodjaeva (2011) cytomeglovirus infection in pregnancy and the : a case presentation . Medical and Health Science Journal, Volume 8, pp. 27-31.
- Christian LM , Iams JD, Porter K, Glaser R. (2012) .Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination . Brain Behav Immun. 26(8):1280-7.
- Di Stefano M, Calabrò ML, Di Gangi IM, Cantatore S, Barbierato M, (2008)In Vitro and In Vivo Human Herpesvirus 8 Infection of Placenta. PLoS ONE 3(12): e4073. doi:10.1371 .
- Gervasi MT ,Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, Hassan SS, (2012) . Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy.J Matern Fetal Neonatal Med. 25(10) doi: 10.3109/14767058.
- Hänel P , Hummel M, Anagnostopoulos I, Stein H. (2001). Analysis of single EBER-positive and negative tumour cells in EBV-harbouring B-cell non-Hodgkin lymphomas. J Pathol.,195(3):355-60.
- Hussein, A.M. Al.Baiati1, Mohammed, A.Muhsin2 and Rebah, N. Jabbar(2014) Seroprevalence of Human CytomegaloVirus (HCMV) in aborted women in Baghdad province.J.Curr. Microbiol.App.Sci 3(2): 97-102.
- Khameneh ZR, Hanifian H, Barzegari R, Sepehrvand N.( 2014 ) Human parvovirus B19 in Iranian pregnant women: a serologic survey. Indian J Pathol Microbiol. 57(3):442-4.
- Lerner MR, Andrews NC, Miller G, Steitz JA (1981). Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci USA.;78:805–809.
- Lu DY, Qian J, Easley KA, Waldrop SM, Cohen C. (2009) Automated in situ hybridization and immunohistochemistry for cytomegalovirus detection in paraffin-embedded tissue sections. Appl Immunohistochem Mol Morphol. 17(2):158-64.
- Majeed(2011). Toxoplasma gondii and cytomegalovirus seropositivity pathogens in high- risk patients in Iraq. Al-Anbar J. Vet. Sci., Vol.: 4 No. (1), ISSN: 1999-6527.
- Margaret L. Gulley and Weihua Tang ( 2008 ) Laboratory Assays for Epstein-Barr Virus-Related Disease. J Mol Diagn. 10(4): 279–292.
- Maysara S.Khalf , Dhammra W.Ahmad , Khalida A.Ibraheem (2012). The Seroprevalence of IgM Among Iraqi Aborted Women Infected with Human Cytomegalovirus. The Iraqi Postgraduate Medical Journal .Vol.11, No.1.
- G Niedobitek ,Teresa Finn, H herbst, J Gerdes, Lena Grillner, f Margareta Landqvist,I Benita Zweygberg , Wigart, H Stein (1988). Detection of cytomegalovirus by in situ hybridization and immunohistochemistry using new monoclonal antibody CCH2: a comparison of methods. J Clin Pathol;41:1005-1009.
- Jenna M. Iwasenko, Jonathan Howard1, Susan Arbuckle3, Nicole Graf, Beverley Hall1, Maria E. Craig , and William D. Rawlinson (2015) .Human Cytomegalovirus Infection Is Detected Frequently in Stillbirths and Is Associated With Fetal Thrombotic Vasculopathy. The Journal of Infectious Diseases Volume 212 Issue 4.
- Oliver A, Overton C. (2014). Diagnosis and management of miscarriage. Practitioner. 258(1771):25-8, 3.
- Sharief, T. (2005). Some immunological studies of missed aborted women infected with cytomegalovirus CMV in Erbil city. Al-Mustansiriyah University .College of science .MSC Thesis.
- Song Zhang , Timothy R.H. Regnault , Paige L. Barker , Kimberley J. Botting , Isabella C. McMillen , Christine M. McMillan , Claire T. Roberts and Janna L. Morrison ( 2015), Placental Adaptations in Growth Restriction, Nutrients, 7, 360-389.
- Staar Mohammad Kadir , Israa Hashim Saadoon. (2012). Cytomegalovirus in pregnancy Second Scientific Conference – Science College – Tikrit University ,Iraq.
- Truong CD, Feng W, Li W, et al. (2009) Characteristics of Epstein-Barr virus-associated gastric cancer: a study of 235 cases at a comprehensive cancer center in U.S. A J Exp Clin Cancer Res; 28:14.
- Weiss LM , Chen YY. (2013) . EBER in situ hybridization for Epstein-Barr Methods Mol Biol.;999: 223-30.
- Yiska Weisblum ,Amos Panet , Zichria Zakay-Rones , Ronit Haimov-Kochman ,Debra Goldman-Wohl , Ilana Ariel , Haya Falk , Shira Natanson-Yaron . (2011). Modeling of Human Cytomegalovirus Maternal-Fetal Transmission in a Novel Decidual Organ Culture . Virol. vol. 85 no. 24 13204-13213.
- Xiao-Xia Yu, Cui -Wei Yao, Jing-Li Tao, Chen Yang, Mian-Na Luo, Shang-Mei Li, and Hua-Feng Liu (2014). The expression of renal Epstein-Barr virus markers in patients with lupus nephritis. Exp Ther Med. 2014; 7(5): 1135–1140.
- Xuan-Hong Tomai(2011). Stillbirth following severe symmetric fetal growth restriction due to reactivation of Epstein–Barr virusinfection in pregnancy. J. Obstet. Gynaecol. Res. doi:10.1111/j.1447-0756.








