Alok K. Pareek*, P. E. Joseph and Daya S. Seth
School of Chemical Sciences, Department of Chemistry, ST. John’s College, Agra - 282 002 India.
Abstract
An efficient synthesis of various substituted azo-coumarin & azo-Schiff base has been synthesized by the condensation of 3-chloro-4-methoxy phenyl malon anilic acid (1a) and different substituted phenyl benzene-azo-salicylaldehyde (5a-5l) with few drops of pyridine as a condensing agent. The constitution of the newly synthesized products has been established on the basis of their spectral studies viz: IR, 1H NMR, Elemental analysis and physical properties as m.p, colour, yield%, molecular formula etc. and their antibacterial activity was carried out against S.aureus and E.coli micro-organisum.
Keywords
Subs. Azo - Coumarins; Azo - Schiff’s Bases; Pyridine; Condensation; Malonic Acid
Download this article as:| Copy the following to cite this article: Pareek A. K, Joseph P. E, Daya S. S. An Efficient Synthesis, Characterization of some Novel 6 -(R) phenyl-azo-coumarin -3- carboxy(3-chloro- 4-methoxy) anilide and 2- hydroxy- 5(R)-phenyl - azo - benzylidine (3- chloro - 4 -methoxy)aniline and their Antibacterial Activity. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Pareek A. K, Joseph P. E, Daya S. S. An Efficient Synthesis, Characterization of some Novel 6 -(R) phenyl-azo-coumarin -3- carboxy(3-chloro- 4-methoxy) anilide and 2- hydroxy- 5(R)-phenyl - azo - benzylidine (3- chloro - 4 -methoxy)aniline and their Antibacterial Activity. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1254 |
Introduction
Many of the synthetic, semi-synthetic, natural molecules containing the coumarin scaffold and included simple coumarin, azo-coumarin, pyrano-coumarin, iso-coumarin, benzo-coumarin have become an important class of heterocyclic compounds in drug research. Azo-coumarin have been reported to have multiple biological activities 1, some azo-coumarin and their derivatives have been reported to possess anti-coagulant2, antibacterial3, antifungal4, antiallergic5, antidiabe-tic6, analgesic7, activities. Azo-schiff’s bases are also important class of heterocyclic compounds and had got wide applications in pharmacology and industrial fields8. Some substituted azo-schiff’s bases and their derivatives have also been reported to possesses antiviral9, anticancer 10, antibacterial11, activities. In this laboratory also a number of substituted azo-coumarins and azo-schiff’s bases and their derivatives have been prepared by various workers12-15.
In continuation of our previous work16-18 in the present study we report here the condensation reactions of 3-chloro-4-methoxy malon anilic acid (1a) with different substituted phenyl benzene-azo-salicylaldehydes (5a-5l) in the presence of pyridine as a condensing agent.
Experimental
Material and Methods
All the chemicals used were obtained from Sigma-Aldrich Company. All the recorded melting points were determined in open capillary tubes and are uncorrected. The purity of final products were checked by TLC using Silica-gel-coated AI-Plates. IR (infrared spectrum) (Kbr-disc method cm-1) were recorded on Perkin-Elmer Spectrum RX-1 FT-IR spectrophotometer at ST. John’s college, Agra. 1H NMR spectra measured on Advance Bruker DRX-300 using solution in DMSO d6. Chemical shifts are given in δ (ppm) and protons signals are indicated as: s = singlet, d = doublet, t = triplet, m = multiplet. Elemental analysis was performed on Elementor Vario EL III. All of the synthesized compounds gave satisfactory results. The physical and analytical properties of synthesized compounds are record-ed in Table-1.
Synthesis of N – (3-chloro- 4-methoxy) phenyl malon anilic acid (1a), N : NI – di – (3 – chloro – 4 methoxy) phenyl malonamide (2a), Ethyl-N-(3-chloro- 4 – methoxy) phenyl malonamate (3a), and N-(3-chloro- 4 -methoxy) phenyl malona-mic acid amide (4a)
To the substituted aniline (3 – chloro – 4 -methoxy; 0.025 mole), diethyl malonate (0.05 mole) was added in the presence of catalyst (DMF), refluxed for about 45-60 minutes, cooling, filtered (1). The solid part was recrystallized by ethanol 99%, on analysis it was identified to be N:NI di-(3-chloro – 4-methoxy) phenyl malonamide (2a). The filtrate (1) of main product was collected in a china dish and concentrated by heating it on boiling water-bath, the coloured mass was treated with 25 ml portion of petroleum ether (100-120OC), recrystallized by petroleum ether several times, it was found to be ethyl N – (3 – chloro – 4 -methoxy) phenyl malonamate (3a). Ethyl N-(3-chloro- 4 – methoxy) phenyl malonamate (3a; 0.01 mole) was dissolved in 15 ml of ethanol in r.b.fla-sk was added 15 ml of liquor ammonia, the flask was tightly corcked vigorously shaken, cooling, filtered, purified by ethanol, it is identified to be N (3 – chloro – 4 – methoxy) phenyl malonamic acid amide (4a). In the filtrate (1) of main product add ethanol (20 ml) with a solution of Na2CO3 (20 ml), and then take the hydrolysis of reaction mixture for about 45 minutes, filtered, to the above filtrate add concentrated HCI drop-wise, thus the solid was separated, filtered,washed with distilled water, recrystallized and it was identified to be N (3-chloro- 4 -methoxy) phenyl malon anilic acid (1a).
Synthesis of 2-hydroxy-5 (R) phenyl benzene-azo benzaldehyde (5a-5l)
To substituted amine (0.025 mole) was diazotised with adding concentrated HCI (8 ml) in distilled water (6 ml) at 0OC in an ice-bath, then add solution of Sodium Nitrite (8 ml) to it with constant stirring, the solution of salicylaldehyde (0.025 mole) in 2N NaOH (20 ml) was added with stirring in to the above diazotised salt solution. The solid is separated out immediately, it was filtered, washed with cold water, recrystallized from absolute ethanol 99%.
Synthesis of 6(R)-phenyl azo-coumarin-3-carboxy (3- chloro – 4- methoxy) anilide (6a-6l) and 2-hydroxy-5(R) phenyl azo-banzylidine-(3-chloro – 4- methoxy) aniline (7a-7l)
A mixture of N (3-chloro-4-methoxy) phenyl malon anilic acid (0.001mole ; 1a) and (0.001mole ;5a-5l) in equi molar quantity (1:1), with few drops of pyridine, the reaction mixture was heated for 4-hours in an oil-bath at 104-110OC, the mixture was first melted and then soon set to a solid, cooling, then the product was digested with the saturated solution of Sodium bicarbonate, washed with water, the azo-schiff’s base was removed by the extraction with hot ethanol (15 ml), the filtrate was concentrated and cooling, the obtained product was identified as substituted azo-schiff’s base (7a – 7l), the residue was recrystallized from hot ethanol several times and identified to be substituted azo-coumarin (6a-6l).
Elemental Analysis for C, H, N of compound (6a) are as 53.90(53.87), 3.12(2.94), 8.25(8.19) and compound (7a) are as 54.16(54.01), 4.01(3.40), 9.77(9.45).
Antibacterial Activity
The newly synthesized compounds were screened for their antibacterial activity against S.aureus and E.coli bacterial strains by filter paper disc diffusion method19-20 was followed by using special Hi-Media Sterile disc code SD-067. Streptomycin was used as a standard drug and compared with results, the compounds were screened at concentration of 25 µg/ml in DMF. The zone of inhibition produced by each compounds was measured in mm and the results of antibacterial activity are given in the Table-3.
Results and Discussion
The infrared spectra of the newly synthesized compounds have been recorded in the frequency region 4000-500 cm-1 and 1H NMR spectral data are recorded in the Table 2.
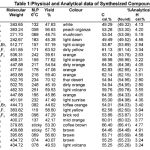 |
Table 1 |
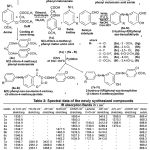 |
Table 2 |
The IR spectrum of compound (1a) shows -NH stretching vibrations at 3415.2 cm-1, -CH2 stretching at 1388.0 cm-1, stretching vibration at 1723.6 cm-1 indicates the -COOH, presence of aromatic -C=C confirm by stretching at 1535.1 cm-1, -CONH confirm by the stretching vibrations at 1655.4 cm-1, linkage at 668.4 cm-1. Above observations are lent support to the assigned structure of compound (1a).
IR spectrum of (2a) shows absorption at 3459.2 cm-1 indicates -NH stretching, absorption at 1388.0 cm-1 (-CH2 str.), while absorption at 1545.0 cm-1(-C=C str.), absorption at 1650.3 cm-1 (-CONH str.), mono substitution at 668.3 cm-1. Hence the assigned structure was in agreement of compound (2a).IR spectrum of (3a) shows absorption at 3412.9 cm-1 indicates (-NH str.), absorption at 1369.9 cm-1 (-CH2 str.), while absorption at 1544.9 cm-1 show -C=C, absorption at 1657.4 cm-1 indicates the presence of -CONH, absorption at 1740.5 cm-1 indicates lactone -C=O, mono substitution at 688.8 cm-1. By above observations the assigned structure was in agreement of the compound (3a).
The IR spectrum of compound (4a) shows -NH stretching vibrations at 3289.4 cm-1, -CH2 str. at 1370.4 cm-1, stretching vibration at 1739.5 cm-1 indicates the -C=O, -C=C confirm by stretching at 1542.3 cm-1, -CONH confirm by the stretching vibrations at 1653.3 cm-1, C-CI linkage at 693.6cm-1. Above observations are lent support to the assigned structure of compound (4a).
The IR spectrum of compound (6a-6c & 6i) shows -NH str. in (3468.2-3413.6 cm-1), -N=N str. in range 1477.5-1462.2 cm-1, str. in range 17 86.4-1727.5 cm-1 indicates the -C=O, (C=C str.) in the range 1562.9-1546.6 cm-1,(-CONH str.) in the range 1664.9-1639.3 cm-1, str.of mono substituti- on in the range 668.4-668.2 cm-1. Above observations are lent support to the assigned structure of compounds (6a-6c & 6i) and other compounds (6d-6h & 6j-6l).
The IR spectrum of compound (7a-7c & 7i) shows -OH str. In (3468.0-3436.1cm-1), -N=N str. in (1484.0-1461.3 cm-1), str. in (2361.6-2361.1 cm-1) indicates the -HC=N, C=C confirm by str. in the range 1548.4-1546.6 cm-1, str. vibrations in the range 668.3-668.2 cm-1 indicates the mono substitution. Above observations of these compounds are lent support to the assigned structure of compounds (7a-7c & 7i) and other compounds (7d-7h & 7j-7l).
The 1H NMR spectra showed singlet at δ 2.500(COOH), 3.813(Ar-H), 3.318(-CH2), 10.160(CONH), confirming the structure of compound (1a), and the spectra of compound (6a) showed signal as singlet at δ 3.333(Ar-CH), 7.169(-C=O), 7.432(-C=C),7.723(-N=N), the above observations confirming the structure of compound (6a) and other compounds (6b-6l), in other compounds the spectra showed signal as singlet at δ 1.227(-C-CH3), 2.010(-OH), 2.500(CH=N), 7.754(-N=N),7.873-7.961(ring), the above results confirm the structure of compound (7b) and other compounds (7a, 7c-7l).
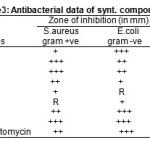 |
Table 3: Antibacterial data of synt. compounds |
Key to symbols: Resistance=R; slighty active = + (inhibi-tion zone 6-9 mm); moderately active = ++ (inhibition zone 9-12 mm);highly active = +++(inhibition zone >12).Key to symbols: Resistance=R; slighty active = + (inhibi-
The antibacterial activity results from Table-3 show that compound (6a,6b,6j) have moderate to highly active against S.aureus and compound (1a,6h,6j) have good activity against E.coli.
Acknowledgements
The Author is thankful to Head, Central Drug Research Institute (CDRI), Lucknow for Spectral (1H NMR) and Elemental analysis and Head of the department of Botany, Raja Balwant Singh College, Agra for biological screening.
References
- D.Egan, R.O.Kennedy, E.Moran, D.Cox, E.proccer and R.D.thornes, Drug Metab. Rev., 22,503(1990)
- M.S.Y.Khan and P.Sharma, Ind. J.Chem., 32B, 374(1993)
- M.S.Y.Khan and P.Sharma, Ind. J.Chem.,34B, 237(1995)
- J.A.A.Miky and A.A.Farrag. Ind.J.Chem., 36B, 357(1997)
- K.Ukawa, Ishiguro, Y.Wada, A.Nohara, Hetero Cycles, 24, 1931(1986)
- D.Heber, Arch.Pharm. , 320,402-406(1987).
- D.Heber,T.Berhaus,J.heterocyclic.Chem.,31,1353(1994)
- P.C.Joshi, Jr and P.C.Joshi,Sr., J.Ind. Chem. Soc., 61, 434(1984)
- A.Das, M.D.Trousdale, S.Renand, E.j.Lien, Antiviral Res., 44, 201(1999)
- J.D.Modi, S.S.Sabxis and C.V.Delizala., J.Mol. Chem., 13, 935(1970)
- S.Goyal and K.Lal., J.Ind. Chem.Soc.,66(7),477-79(1989)
- O.P.Singhal and P.I.Ittyerah, J.Ind. Chem.Soc., 42, 616-618(1965).
- D.S.Seth and B.C.Banerjii,Agra Uni.J.Res.Sci., 27, 107-110(1978).
- S.Sharma, Ph.D.Thesis, Agra Univ.,Agra(1992)
- G.Saxena, A.Choudhary, A.Naqvi,S.Khan and D.S.Seth, Orin.J.Chem.,Vol.23(2),683-686(2007)
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., Vol.25(1), 195-198(2009)
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., Vol.25(3), 681-685(2009)
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., Vol.25(4), 1149-1152(2009)
- R.Cruickshank, J.P.Duguid, B.P.Marion and RHA, Medicinal Microbiology,12th Edn.,2,196-202(1975)
- C.A.Barry, J.L.Joyce, P.A.Adams, J.E.Benner. Am.J.Cli.Pathol., 59, 693(1973)







