Shirin Pazuki1, Alireza Kamali1*, Nasim Shahrokhi1 and Mehri Jamilian2
1Department of Anesthesiology, Arak University of Medical Sciences, Arak, Iran.
2Department of Gynecology and Obstetrics, Arak University of Medical Sciences, Arak, Iran.
Corresponding Author E-mail: alikamaliir@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1039
Abstract
Pain is a complex medical issue. Lack of postoperative pain relief adversely affects the physiological, metabolic and mental conditions of patients. Duration of analgesia could increase by using additional supplements. This study aimed to compare the effects of intrathecal midazolam and tramadol with the conventional method of postoperative pain and shivering control after elective cesarean section. This double-blind randomized clinical trial was conducted on 210 women aged 20-35 years with ASA class I and II who were candidates for elective cesarean section. Subjects were randomly divided into three groups of midazolam, tramadol and control to receive hyperbaric lidocaine and midazolam (2 mg), tramadol (25 mg), and normal saline (5 cc), respectively. Using the visual analogue scale (VAS), postoperative pain score and analgesia duration were assessed and compared between the groups. Moreover, scores of shivering during and after the surgery were compared between the groups. According to the results of Kruskal-Wallis, mean score of postoperative analgesia duration was significantly higher in the tramadol group compared to the other groups, while it was higher in the midazolam group compared to control subjects (P<0.001). Moreover, mean consumed dose of analgesics at 24 hours after surgery was significantly lower in the tramadol group compared to the other groups, while it was significantly lower in the midazolam group compared to control subjects (P<0.001). At different time intervals, postoperative shivering was significantly lower in the tramadol group compared to the other groups, while it was significantly lower in the midazolam group compared to control subjects (P<0.01). According to the results of this study, intrathecal midazolam and tramadol supplementary to lidocaine 5% could increase analgesia duration and reduce postoperative shivering after cesarean section, and midazolam was more effective than tramadol in this regard. .
Keywords
Midazolam; Postoperative complications; Spinal anesthesia; Tramadol
Download this article as:| Copy the following to cite this article: Pazuki S, Kamali A, Shahrokhi N, Jamilian M. Comparison of the Effects of Intrathecal Midazolam and Tramadol with the Conventional Method of Postoperative Pain and Shivering Control after Elective Cesarean Section. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Pazuki S, Kamali A, Shahrokhi N, Jamilian M. Comparison of the Effects of Intrathecal Midazolam and Tramadol with the Conventional Method of Postoperative Pain and Shivering Control after Elective Cesarean Section. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11714 |
Introduction
Pain is a common postoperative complication, and anesthesiologists primarily aim at the reduction and control of pain after surgical operations (1). Despite the importance of pain control during surgery, new methods of analgesia for postoperative pain relief have attracted the attention of many researchers. Effective postoperative pain control results in patient satisfaction, decreased length of hospital stay, and reduction of treatment costs (2).
Cesarean section is a freqeunt surgery among women, and postoperative analgesia is an important issue for both patients and surgeons. Statistics suggest that general anesthesia after cesarean section is associated with a higher risk of mortality compared to local ansthesia (1). Therefore, local anesthesia is preferable for maintaining the health of the mother and infant.
Today, various techniques are availabe for local ansthesia, the effectiveness of which has been confirmed in pain relief after cesarean section (2, 3). As such, use of these techniques is on the rise due to their easy application and high efficacy (2).
Some of the common complications of anesthesia are pain and shivering, respiratory depression in mother and infant, and nausea and vomiting. Given the importance of infant care by the mother immediately after recovery, use of analgesic drugs with limited side effects and effective anesthetic methods must be priritized in these women (4).
Pain is a complex and multifaceted phenomenon, which is infleunced by various parameters (4, 5). Duration of analgesia could increase through the use of additive compounds (e.g., epinephrine, opioids, neostigmine, midazolam and clonidine) in local anesthetics. Furhermore, these compounds are able to diminish other postoperative complications, such as shivering (6, 7).
According to the literature, interathecal administration of midazolam with local anesthetics is effective in regional and neuraxial anesthesia for postoperative pain control. Midazolam has been shown to have remarkable analgesic effects with limited side effects (4). On the other hand, tramadol, which is an atypical synthetic drug, exerts central analgesic effects, while its intrathecal administration could reduce postoperative pain and shivering (8).
This study aimed to compare the effects of intrathecal midazolam and tramadol with the conventional method of postoperative pain and shivering control after elective cesarean section.
Materials and Methods
This interventional, double-blind randomized clinical trial was conducted on 210 women aged 20-35 years with the American Society of Anesthesiologists (ASA) physical status class I and II, who were candidates for elective cesarean section.
Sample population consisted of 210 participants who were randomly divided into three groups of midazolam, tramadol and control. An anesthesiologist examined candidates for non-emergency cesarean section referring to Taleghani Hospital of Arak, Iran. After explaining the objectives of the study and obtaining informed consent, eligible women were enrolled in the study.
Random allocation of the participants was performed using a random number table. Estimation of the level of pain was based on a numerical rating scale using the 10-centimeter spectrum of the visual analogue scale (VAS). Patients were asked to mark number 10 in case of severe pain and zero to indicate the absence of pain, while other numbers were marked depending on the intensity of postoperative pain.
Inclusion criteria of the study were as follows: 1) age of 20-35 years; 2) ASA class I and II; 3) no drug addiction; 4) lack of cardiac, renal and liver diseases; 5) no allergies to local anesthetics and 6) consent to receive spinal anesthesia. Exclusion criteria were the patients in whom spinal anesthesia failed and was replaced with general anesthesia, those undergoing spinal anesthesia more than twice, and patients with surgery duration of more than 90 minutes.
Initially, participants were divided into three groups based on the random number table. Candidates aged 35-55 years were administered with crystalloid (3-5 ml per kilogram of body weight) as the compensatory volume expansion. Following that, while patients were in a sitting position, spinal anesthesia was induced by a resident under sterile conditions through injection with a 25-gauge spinal needle between L5-L4 or S1-L5 lumbar regions using 75 mg of hyperbaric spinal lidocaine 5% (Rion Pharma, manufacturing number: 1295235).
Patients were equally divided into three groups of midazolam (2 mg=0.5 cc), tramadol (25 mg=0.5 cc) and control (0.5 cc normal saline) (70 subjects per each group). In this study, 2 cc of the solution was injected into the intrathecal space, and surgical operation was performed five minutes after spinal anesthesia reached a T4 level. During the surgery, oxygen flow was set at the speed of 5-6 lit/min thorough a mask, and vital signs of the patients were measured and recorded simultaneously, including blood pressure, heart rate and arterial blood oxygen saturation.
Pain intensity scores of all the patients were evaluated based on VAS during recovery, as well as 4, 12 and 24 hours after the surgery. Moreover, trained nurses who were blinded to the medication use of patients verified the mean prescribed dose of analgesics within 24 hours after the surgery. Additionally, intensity of shivering during the surgery, recovery and four hours after the surgery was recorded in prepared checklists.
This study was performed using a double-blind design, so that patients were justified on their random allocation to each study group. Furthermore, they were assured that none of the medications would cause inconvenience or side effects, and the participants were blinded to the type of the injected anesthetics as well.
A resident performed the administration of spinal anesthesia, and an intern determined the level and score of postoperative pain and mean dose of the consumed analgesics. These individuals were blinded to the type of intrathecal anesthetics. Before injection, researchers and anesthesiologists prepared the anesthetics, and a resident who was unaware of the type of drugs carried out the intrathecal injection.
Furthermore, residents who were blinded to the type of anesthetics completed the questionnaires, which consisted of data on the pain intensity score, vital sign changes, mean dose of the consumed analgesics within 24 hours after the surgery, and duration of analgesia.
Data analysis was performed in SPSS version 16 using the Kruskal-Wallis test and analysis of variance (ANOVA). Sample size was determined at 210 subjects selected via simple random sampling, who were equally classified into three groups (70 women per each group). Using random number tables, participants were matched in terms of age, type of surgery, same surgeon in all operations, gender (all female), and no history of previous gynecological surgery. It is noteworthy that our participants had the same surgeon, and duration of surgery was similar in all the subjects according to the exclusion criteria of the study.
Ethical considerations
This study was performed in compliance with the principles and ethical codes of Arak University of Medical Sciences in all the stages. Study procedures and requirements were explained to the patients, and written informed contest was obtained prior to participation.
Tramadol and midazolam have been widely used as complementary drugs for spinal anesthesia, and no spinal complications have been reported in this regard. This article was extracted from a research project.
Result
Mean age of the participants in this study was 25.05±5.9 years, which was similar in all the groups. According to within-subjects test, there was no significant difference between the groups in terms of age (P>0.05). Mean arterial pressure before spinal anesthesia was estimated at 82.2±1.4 mmHg in all the groups, which was indicative of no significant difference in this regard (P>0.05).
Before spinal anesthesia, mean heart rate was calculated at 104.5±7.2 bpm in all the groups, which was indicative of no significant difference in this regard (P>0.05). According to our findings, mean duration of analgesia in patients of the tramadol, midazolam and control groups was 192.5±12.2, 111.3±16.6 and 86.1±9.9 minutes, respectively. According to the results of Kruskal-Wallis test, study groups had a significant difference in terms of the duration of analgesia. As such, postoperative analgesia duration was significantly longer in the tramadol group compared to the other two groups, while it was longer in the midazolam group compared to placebo subjects (P<0.001).
At 24 hours after the surgery, results of Kruskal-Wallis test indicated that mean dose of consumed analgesics was significantly lower in the tramadol group compared to the other groups, while it was lower in the midazolam group compared to placebo subjects (P<0.01). In patients of the tramadol group, mean dose of diclofenac suppository was 8.2±2.4, while it was 9.9±2.7 in the midazolam group and 11.2±3.3 in control subjects.
According to the results of ANOVA, mean score of postoperative pain during recovery and 4, 12 and 24 hours after the surgery was significantly lower in the tramadol group compared to the other two groups, while it was significantly lower in the midazolam group compared to control subjects (P<0.01).
With regard to postoperative shivering, study groups had a significant difference, as the mean score of shivering during surgery was lower in patients of the tramadol group compared to the other two groups, while it was lower in the midazolam group compared to control subjects (P<0.01). However, this was not observed during recovery and four hours after the surgery (P<0.02). On the other hand, nausea and vomiting were more prevalent among the patients of tramadol and midazolam groups compared to placebo subjects, which required special care and attention.
 |
Figure 1: Mean blood pressure of patients in tramadol, midazolam and control groups before spinal anesthesia |
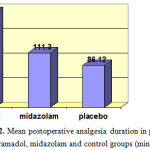 |
Figure 2: Mean postoperative analgesia duration in patients of tramadol, midazolam and control groups (min) |
Findings of this study were indicative of a significant difference in the pain scores of patients in the three groups at 4, 12 and 24 hours after the surgery. Correspondingly, pain score of the tramadol group was significantly lower compared to the other two groups, while the pain score of patients in the midazolam group was significantly lower compared to control subjects.
Table 1: Pain scores of patients in tramadol, midazolam and control groups during recovery
| Statistical test/P-value | Pain score | Groups | Time |
|
Kruskal-Wallis ≤0.01 |
0.65±0.23 | Tramadol |
Recovery |
| 1.4±0.81 | Midazolam | ||
| 2.72±0.95 | Placebo |
Table 2: Pain scores of patients in tramadol, midazolam and control groups (4 hours after surgery)
| Statistical test/P-value | Pain score | Groups | Time |
|
Kruskal-Wallis ≤0.02 |
3.5±1.1 | Tramadol |
4 hours postoperative |
| 3.7±1.2 | Midazolam | ||
| 7.3±2.7 | Placebo |
Table 3: Pain scores of patients in tramadol, midazolam and control groups (12 hours after surgery)
| Statistical test/P-value | Pain score | Groups | Time |
|
Kruskal-Wallis ≤0.01 |
2.6±4.5 | Tramadol |
12 hours postoperative |
| 2.9±3.6 | Midazolam | ||
| 4.8±6.6 | Placebo |
Table 4: Pain scores of patients in tramadol, midazolam and control groups (24 hours after surgery)
| Statistical test/P-value | Pain score | Groups | Time |
|
Kruskal-Wallis ≤0.02 |
0.95±0.78 | Tramadol |
24 hours postoperative |
| 1.1±2.1 | Midazolam | ||
| 3.3±3.8 | Placebo |
Discussion
According to the results of the present study, addition of midazolam or tramadol to lidocaine 5% for postoperative pain control in spinal anaesthesia could result in the discovery of alternative medications with fewer side effects to increase the analgesia duration and decrease pain intensity after caesarean section. This has been a major concern among anaesthesiologists.
Increasing the duration of analgesia prevents several postoperative complications, such as atelectasis, urinary retention, prolonged hospital stay and increased healthcare costs. Therefore, replacement of conventional analgesic methods with new pain-relieving compounds is of paramount importance. Some of the most effective drugs used in previous studies in this regard are ketamine, midazolam, neostigmine and tramadol.
In the current study, patients in the three groups had no significant difference in terms of age, and mean age of the participants was 24.05±5.9 years. With respect to the duration of postoperative analgesia, a significant difference was observed between the groups, as the duration of analgesia was significantly longer in the tramadol group compared to the other two groups, while it was longer in the midazolam group compared to placebo subjects.
Furthermore, mean dose of consumed analgesics at 24 hours after surgery was significantly lower in the tramadol group compared to the other two groups, while it was lower in patients of the midazolam group compared to control subjects. In women of the tramadol, midazolam and control groups, mean dose of diclofenac suppository at 24 hours after surgery was 2.4±8.2, 2.7±9.9 and 3.3±11.2, respectively.
Mean score of pain intensity during recovery and at 4, 12 and 24 hours after surgery was significantly lower in the tramadol group compared to the other two groups, while it was lower in the midazolam group compared to control subjects.
Similar studies have also suggested that intrathecal addition of midazolam and tramadol to lidocaine 5% could effectively increase the duration of postoperative analgesia and reduce pain intensity. For instance, in a research conducted in India, Sen A et al. (2001) evaluated the effects of midazolam (2 mg) on postoperative pain control, vital signs of the mother and one- and five-minute Apgar scores of infants in 40 women.
In the mentioned study, all the subjects received 1.5 cc of lidocaine 5% and intrathecal midazolam (2 mg). According to the results, intrathecal administration of midazolam could effectively control the pain after cesarean section, while exerting anti-nausea and tranquilizing effects (31).
In a clinical trial performed by Prakash et al. (2006), 60 pregnant women undergoing elective cesarean section receiving spinal anesthesia were examined. All the patients received 2 cc of bupivacaine 0.5%; the first group received 1 mg of midazolam in addition to bupivacaine, and the second group received 2 mg of midazolam.
According to the results of the mentioned research, mean duration of postoperative analgesia in the control group and experimental groups one and two was 3.8±0.5, 4.3±0.7 and 6.1±1.0 hours, respectively (P<0.001). Moreover, need for diclofenac administration was significantly lower in the second group compared to the first group and control subjects.
On the other hand, nausea and vomiting was significantly higher in the control group. As stated by the researchers, 2 mg of midazolam could induce an average local anesthesia duration and be used as an adjuvant therapy to control nausea and vomiting after cesarean section (33). These findings are in congruence with the results of the present study. However, frequency of nausea and vomiting was observed to be higher in patients of the tramadol and midazolam groups compared to control subjects in our research, which might be due to the differences in the type of drugs and manufacturers.
Other studies in this regard have also mentioned the higher frequency of nausea and vomiting after surgery in experimental groups compared to control subjects. For instance, Shukla U et al. (2011) compared the effects of clonidine and tramadol on the management of postoperative shivering after spinal anesthesia. Researchers examined 80 patients and reported that clonidine was more effective than tramadol in controlling postoperative shivering. In the mentioned study, complete remission of shivering symptoms in clonidine and tramadol groups occurred at 2.54±0.76 and 5.01±1.02 hours, respectively. Furthermore, response rate was estimated at 97.5% in the clonidine group and 92.5% in the tramadol group (P=NS) (34).
In another study in this regard, Mohata M et al. (2009) compared the effects of three different doses of tramadol (1, 2 and 3 mg/kg), pethidine (0.5 mg/kg) and normal saline on the control of postoperative shivering in patients undergoing elective abdominal surgery with general anesthesia. According to the findings, all tramadol doses were effective in the control of postoperative shivering with no side effects. Therefore, use of this analgesic drug was recommended for the alleviation of postoperative shivering (14).
Another research in this regard was conducted by Honarmand et al. (2008) in Isfahan University (Iran) to compare the effects of prophylactic midazolam, ketamine and combination of both drugs on the control of shivering after spinal anesthesia in 120 orthopedic patients.
According to the results, level of postoperative shivering was 60% in the control group, 50% in the midazolam group, 23.3% in the ketamine group, and 3.3% in patients administered with both ketamine and midazolam. Evidently, frequency of postoperative shivering was significantly lower in the last group compared to the other patients. Moreover, number of patients with shivering score of lower than three was significantly higher in the control group compared to the other groups (6).
Based on the findings of the aforementioned studies, it could be inferred that tramadol and midazolam increase the duration of analgesia and decrease the level of pain and shivering postoperatively, which is consistent with the results of the present study. It should be noted that none of the mentioned studies compared the effects of these drugs, while in our research, we compared the effects of additive tramadol and midazolam to lidocaine 5%. Our findings were indicative of the higher effectiveness of tramadol in controlling postoperative pain and shivering.
In conclusion, it is recommended that similar studies in this regard be conducted on larger sample sizes to confirm our findings. Moreover, it is suggested that postoperative nausea and vomiting due to the use of these drugs be further investigated.
Limitations of the Study
One of the limitations of the present study was the disagreement of some patients with spinal anesthesia or addition of complementary drugs to their regimen; these individuals were excluded from further experimentation. The only postoperative complication in the current study was the slight reduction of the blood pressure in patients of the midazolam and tramadol groups. This was followed by the higher mean heart rate of the participants in these groups due to the higher ephedrine dose. This complication could be controlled through the adequate hydration of patients before surgery.
Acknowledgements
Hereby, we extend our gratitude to all the patients and personnel of Taleghani Hospital of Arak for assisting us in this research project. We would also like to thank the Vice Chancellor of Research at Arak University of Medical Sciences for the financial support of this study.
References
- Stamer U, Schneck H, Grond S, Wulf H. Surveys on the use of regional anaesthesia in obstetrics. Curr Opin Anaesthiol. 1999; 12:565-71.
- Ng k, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004:CD003765.
- Practice guidelines for obstetrical anaesthesia: a report by the American Society of Anaesthesiologists Task Force on Obstetrical Anaesthesia. Anaesthesiology. 1999; 90:600-611.
- Son A, Rudra A, Sarkar SK , Biswas B. Intrathecal Midazolam for postoperative pain relief in caesarean section delivery J Indian Med Assoc 2001; 19(12) : 683-4, 686.
- Siddigi R, Jafari SA . Maternal satisfaction after spinal anesthesia for caesarean deliveries. J coll physicians sury Pak. 2009 feb; 19(2) : 77-80.
- Honarmand A and safavi M.R. comparsion of phrophylactic use of midazolam, ketamin, and ketamin plus midazolam for prevention of shivering during regional anesthesia: a randomized double – blind placebo controlled trial . Brithish Journal of Anesthesia 2009 , 101(4) : 557-62.
- Joshi N, Shhavi C.S, Saxena A.K. Comparative Evaluation of Analgesic Efficacy of Intrathecal Clonidine with Bupivacaine versus Intrathecal Midazolam with Bupivacaine in Patients Undergoing Cesarean Section. Indian J PAIN. 2010;24: 41-45.
- Asim Mahmood M and Richard M. Progress in shivering control. Journal of the Neurological science 2007, 261: 47-54.
- Larry d. crowley M.B, M.R.C.P. shivering and Neuroaxial Anesthesia . Regional Anesthesia and Pain medicine 2008, 241-252.
- Goodchild GS, Serrao JM. Intrathecal midazolam in rats evidence os spinally mediated analgesia. Br J Anaesth. 1987; 59:563-70.
- Yaksh TL, Reddy SVR. Studies in primates on the analgesic effects associated with intrathecal actions of opiates, ±2 adrenergic agonist and baclofen. Anesthesiology 1981; 54:451-7.
- De Whitte, Sessler DI. Perioperative shivering: Physiology and Pharmacology. Anaesthesiology. 2002;96:467–84.
- Miller Ronald D, Eriksson lurs L, Fleisher Lee A, weine – kronirhJ, Young w. Miller’s Anesthesia. 7th ed. churchil living stone. 2010.
- Mohta M, Kumari N, Tyagi A, Sethi A.K, Agarwal D, Singh M. Tramadol for prevention of postanaesthetic shivering: a randomised double-blind comparison with pethidine. Anaesthesia. 2009;64: 141-146.
- Bhatnagar S, Saxena A, Kannan TR, Punj J, Panigrahi M, Mishra S. Tramadol for postoperative shivering: A double blind comparison with Pethedine. Anaesth Intensive care. 2001;29:149–54.
- Katyal S, Tewari A. Shivering: Anesthetic Considerations. J Anaesth Clin Pharmacol. 2002;18:363–76.
- Sessler Daniel I. Temperature Monitoring. In: Millar RD, editor. Textbook of Anaesthesia. 5th ed. New York: Churchill Livingstone Inc; 1994. pp. 1367–89.
- Ho KM, Ismail H. use of inthrathecal midazolam to improve perioperative analgesia. a meta – analysis. Anesth Intensive care. 2008; 30(3): 365-73.
- Olkala KT, A honen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol 2008; (182):335-60.
- Kuriyama K, Yoneda Y. Morphine induced alterations of gaminobutyric acid and taurine contents and L-glutamate decarboxylase activity in rat spinal cord and thalamus:possible correlates with analgesic action of morphine. Brain Res. 1998; 148:163-179.
- Mohler H, Okada T. The bezodiazepine receptor in normal and pathological human brain. Br J Psychiatry. 1987; 133:261-268.
- Niv D, Whitwam JG, Loh L. Depression of nociceptive sympathetic reflexes by the intrathecal administration of midazolam. Br J Anaesth. 1983; 55:541-547.
- Boulter N, Serrao JM, Gent JP, Goodchild CS. Spinally mediated antinociceptive following intrathecal chlordiazepoxide further evidence for a benzodiazepine spinal analgesic effect. Eur J Anaesthesiol. 1991; 8:407-411.
- Duncan MA, Suvage J. Tucker AP. Prospective audit comparing intrathecal analgesia (in corporating midazolam ) with epidural and intravenous analgesia after major open abdominal surgery. Anesthesia intensive care. 2007; 35(4): 556-62.
- Kurz A, Sessler DI, Annadata R, Dechert M, Christensen R, Bjorksten AR. Midazolam minimally impairs thermoregulatory control. Anesth Analg. 1995; 81-393-8.
- Gover V.K, Manajan R, Gill K.D. Influence of midazolan on postoperative shivering. Journal of Anesthesiology clinical pharmacology. 2002;377-382.
- Alhashemi JA, koki AM. Effect of intrathecal tramadol administration on postoperative pain after transurethral resection of prostate. Br J Anesth. 2003 oct; 91(4): 536-40.
- Mathews S, Mulla A, Varaghese PK, Radim K, Mumtaz S. Post anaesthetic shivering- a new look at tramadol. Anaesthesia. 2002; 57: 394-8.
- De Witte J, Rietman GW, Vandenbroucke G, Deloof T. Post-operative effects of tramadol administered at wound closure. European Jornal of Anaesthesiology. 1998; 15:190-5.
- Shipton EA. The further. In: Shipton EA, ed. Pain- Acute and Chronic. London:Arnold. 1999:344-6.
- Sen A , Rudra A , Sarkar SK , Biswas B. Intrathecal midazolam for postoperative pain relief in caesarean section delivery. J Indian Med Assoc. 2001; 99(12):683-4, 686.
- Samimi Sade S, Davari Tanha F, Sadeghi S. Prevention of Postoperative Nausea and Vomiting by Administration of Sub Hypnotic Doses of Propofol and Midazolam during Spinal Anesthesia for Cesarean Section. Jornal of Family and Reproductive Health. 2010; 4(4):175-8.
- Prakash S, Joshi N, Gogia A.R, Prakash SU, Singh R. Analgesic Efficacy of Two Doses of Intrathecal Midazolam With Bupivacaine in Patients Undergoing Cesarean Delivery. Regional Anesthesia and Pain Medicine.2006;31(3): 221–226.DOI: 1016/j.rapm.2006.02.006
- Shukla U, Malhotra K and Prabhakar T. A comparative study of the effect of clonidine and tramadol on post-spinal anaesthesia shivering. Indian J Anaesth. 2011 May-Jun; 55(3): 242–246. doi: 4103/0019-5049.82666.
- Heid F, Grimm U, Roth W, Piepho T, Kerz T, Jage J. Intraoperative tramadol reduces shivering but not pain after remifentanil-isoflurane general anaesthesia. A placebo-controlled, double-blind trial. Eur J Anaesthesiol. 2008 Jun;25(6):468-72. Epub 2008 Feb 21.
- Claahsen-van der Grinten HL, Verbruggen I, Van den Berg PP, Sporken JM, Kollee LA. Different pharmacokinetics of tramadol in mothers treated for labor pain and in their neonates. Eur J Clin Pharmacol. 2005; 61(7):523-9.
- Siddik-Sayyid S, Aouad-Maroun M, Sleiman D, Sfeir M, Baraka A. Epidural tramadol for postoperative pain after cesarean section. Can J Anaesth 1999 Aug; 46(8): 731-5.








