Manuscript accepted on :June 19, 2010
Published online on: 20-11-2015
Plagiarism Check: Yes
Archana P. Ramteke and Mandakini B. Patil*
University Department of Biochemistry, RTM, Nagpur University, Nagpur - 440 033 India.
Abstract
Human pathogens were treated with purified extract of calyx of Tridax procumbans L. and its effect on growth of the bacteria was observed. The extract had the ability to agglutinate the bacteria and no growth of the bacteria on the solid growth medium was seen when grown at appropriate condition, reflecting the properties to be of pharmaceutical importance.
Keywords
Pathogenic bacteria; Proteins; Tridax procumbans L
Download this article as:| Copy the following to cite this article: Ramteke A. P, Patil M. B. Augmentation of the Growth of Pathogenic Bacteria by Proteins of Tridax Procumbans L. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Ramteke A. P, Patil M. B. Augmentation of the Growth of Pathogenic Bacteria by Proteins of Tridax Procumbans L. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1133 |
Introduction
Plant proteins are reported as the most widely used constituents in biomedical research. Pathogenic organisms often attach to human epithelial or erythrocytes cell surfaces via Plant protein-glycan interactions1. Bacterial species are important opportunistic pathogens having the ability to cause diarrhoea, dysentery and extra-intestinal infections2. Many pathogenic bacterial species can be distinguished by their carbohydrate rich surface antigen, which is referred to as glycoepitopes. These glycoepitopes are associated with capsular polysaccharides3.
Plant proteins are used for direct clumping of microorganisms in microbiology. Clumping of Gram-negative Diplococcus by Wheat germ agglutinin (WGA) has been considered as positive test for N. gonorrhea. Similarly, Bacillus anthracis can be identified by clumping with soybean agglutinin4. Plant proteins are able to selectively clump Pyogenic cocci. Groups A, B, C, F and G streptococci can be readily distinguished by Plant proteins, however, Salmonella genus was unable to react with Con A.5.
The interaction between most Plant proteins and microbial surfaces are due to the glycoconjugates of microbial surfaces which is a potential site for clumping. Some Plant proteins were able to selectively aggregate bacteria6.
Medicinal plants are routinely exploited for the study of their conventional uses through the authentication of pharmacological effects and can be natural complex sources of new anti-infectious agents. The present study aimed at evaluating the agglutination activity of plant Tridax procumbans calyx protein against bacterial strains isolated from human infection following the agglutination assay of Tridax procumbans calyx protein with the pathogenic bacteria and to check the growth of clumped bacteria on the growth medium by evaluating the result with suitable statistical analysis.
For our convenience Tridax procumbans calyx protein has been designated as TPCP.
Materials and Methods
Plant protein
Calyx of the plant T procumbans L, a wild medicinal plant of the family compositeae, was used as the source of plant protein7.
Reagents and Glassware
Papain, BSA, guar–gum, D – glucose, D – galactose, sucrose, raffinose, N – Acetyl – D – glucosamine, D – glucosamine hydrochloride, a – pNPG, b – oNPG, Acrylamide, Bis – acrylamide, b – mercaptoethanol, coomassie brilliant blue (R 250), were obtained from Sigma chemicals, St Louis, M.O., U.S.A. Distilled water, glass wares like petri dishes, pipettes, glass rods, beakers, test tubes, etc. were sterilized in autoclaved at 121oC at 15 lbs pressure for 15 min. Other chemicals were of analytical grade.
Bacterial isolates
The bacterial isolates like Enterococci faecalis, Escherichia coli, Klebsiella pneumonie, Morganella species, Proteius mirabilis, Pseudomonas aeruginosa, Providentia species, Salmonella typhi, Salmonella paratyphi- A and Salmonella paratyphi-B were obtained from Department of Microbiology, Indira Gandhi Government Medical College, Nagpur and were grown on specific growth medium.
Purification of Plant Protein
Preparation of crude extract
Calyx of 45 days old plants (100 g) grown in the garden of University Department of Biochemistry, Nagpur University, Nagpur, India7, were collected, washed four times under the tap water and two times with distilled water. After soaking between the folds of filter paper, they were homogenized in 1L of 1M NaCl and kept on shaker at 4°C for 2 hours, to ensure proper extraction of the Plant protein. The extract was passed through two layers of cheese cloth and the filtrate was centrifuged at 12000 rpm (9668g) at 4°C for 30 min. The supernatant obtained, designated as crude extract, was used for isolation and characterization of TPCP as described elsewhere8.
Polyacrylamide Gel Electrophoresis
The homogeneity of TPCP was tested on SDS – PAGE. Molecular weight of the purified plant protein was determined by the method of Weber and Osborn (1969). Acrylamide concentration for the running separator gel was 7.5% and that of the stacking gel was 2.5%, marker proteins such as Carbonic anhydrase 29kD, Trypsin 23kD, Myoglobin 17.2kD, Cytochrome C – 12.3kD, were used as standard proteins. After electrophoresis the gels were stained with 1% coomassie brilliant blue (R – 250) prepared in destaining solution containing 7% acetic acid. The gels were destained in 7% acetic acid9, 10.
Protein Estimation
Proteins were estimated by the method of Lowry et al., (1951). Bovine serum albumin was used as standard protein (Lowry et. al., 1951)11.
Growth Of Bacterial Strains
The pathogenic bacteria were grown on appropriate medium on test tube slants (Table 1).
Preparation Of The Bacterial Growth Medium
Blood Agar medium
Peptone 2%
Beef extract 1%
NaCl 0.5%
pH 7.4
Agar 3%
Autoclave upto 560
Add 10% Human or Sheep Blood
Macconkeys Agar medium
Peptone 2%
Na Taurochlorate 0.5%
pH 7.4
Agar 3%
Red Indicator 0.1% aquas solution
Autoclave upto 560
Add 1% lactose
Shigella Agar (Ready to use Himedia)
Nutrient Agar medium
Yeast extract 5g
Peptone 10g
pH 6.8
Agar 15g
Distilled water 1L
Autoclave upto 560
Bacterial Agglutination
Bacterial agglutination assay using purified TPCP was performed by the method of Zanette, et. al., (2000)14. The Bacterial agglutination titre was defined as the reciprocal of the highest dilution that was able to induce visible agglutination12.
Qualitative determination of clumping of bacteria
This method is a quick screening method for qualitative determination of agglutination. A bacterium was carefully removed from the test tube slants and was suspended in PBS. One drop of 50μl of heavy bacterial suspension (OD=1) was mixed on a slide with fifty μl of TPCP (250 μg per ml), respectively12. Clumping of bacteria with TPCP is considered as positive test. One drop of 50μl added to 50μl of normal saline was considered as control.
Quantitative determination of the clumping of bacteria
The agglutination titre was determined using microtitre plate. The agglutination of bacteria by TPCP was determined by incubating fifty μl plant proteins (250 μg per ml) followed by serial dilution. Fifty μl of bacteria (OD=1) were added to each well separately. After gentle shaking of the reaction mixture (Plant protein + bacteria) was placed on a rotary shaker (50 rpm) for 10 min, the cells were allowed to settle for 45 minutes and the agglutination in the wells were monitored microscopically. Controls were run simultaneously12.
The Bacterial agglutination titer was defined as the reciprocal of the highest dilution of the T. procumbans plant protein extract that yielded visible agglutination activity. One Bacterial agglutination unit (BAU) was defined as the amount of plant protein extract, which causes complete agglutination under the experimental conditions12.
Table 1: Growth medium for pathogenic bacteria
| No | Name of microorganism | Required Growth medium |
| 1 | Enterococci faecalis | Macconkeys Agar |
| 2 | Escherichia coli | Macconkeys Agar |
| 3 | Klebsiella pneumonie | Macconkeys Agar |
| 4 | Morganella species | Macconkeys Agar |
| 5 | Proteius mirabilis | Macconkeys Agar |
| 6 | Pseudomonas
aeruginosa |
Macconkeys Agar |
| 7 | Providentia | Macconkeys Agar |
| 8 | Salmonella typhi | Macconkeys Agar |
| 9 | Salmonella paratyphi- A | Macconkeys Agar |
| 10 | Salmonella paratyphi-B | Macconkeys Agar |
| 11 | Salmonella entritidis | Macconkeys Agar |
| 12 | Serratia species | Macconkeys Agar |
| 13 | Shigella dysenteria | Shigella Agar |
| 14 | Staphylococcus aureus | Blood Agar |
| 15 | Vibrio cholera | Macconkeys Agar |
Growth of clumped bacteria on solid medium
The appropriate agar plates were prepared with specific growth medium as all bacteria were pathogenic and they require specific growth medium. The agar plates were divided into four equal parts. The clumped bacteria by TPCP were determined by incubating fifty μl lectin (250 μg per ml) and fifty μl of bacteria (OD=1) were mixed separately. After gentle shaking, the reaction mixture (lectin + bacteria) was inoculated on agar medium for 24h at 37oC. The bacterial growth was observed on three successive days visually. Controls were run simultaneously13.
Table 2: Characteristics of pathogenic bacteria used for agglutination with TPCPa
| Sr. no. | Name of microorganism | Agglutination | Gram
-ve/+ve |
Cell wall composition | Resulting infection |
| 1 | Enterococci faecalis | + | – | Mannose
Specific |
Bacillary dysentery |
| 2 | Escherichia coli | + | – | Mannose
Specific require Ca+ + |
Urinary tract meningitis
in children Pneumonia, Urinary tract. |
| 3 | Klebsiella pneumonie | – | – | Mannose
Very large capsule |
Pneumonia |
| 4 | Morganella species | + | – | Urinary tract infection
Wound infection |
|
| 5 | Proteius mirabilis | + | – | Urinary tract infection
Wound infection |
|
| 6 | Pseudomonas
aeruginosa |
+ | – | Galactose | Urinary tract, wounds,
Burns, lung infection. |
| 7 | Providentia species | + | – | Diarrhea
Urinary tract infection Wound infection |
|
| 8 | Salmonella typhi | – | – | Mannose | Typhoid fever |
| 9 | Salmonella paratyphi- A | – | – | Mannose | Paratyphoid fever |
| 10 | Salmonella paratyphi-B | – | – | Mannose | Paratyphoid fever |
| 11 | Salmonella entritidis | – | – | Mannose | Typhoid fever |
| 12 | Serratia species | + | – | Meningitis Pneumonia,
|
|
| 13 | Shigella dysenteria | + | – | Mannose | Bacillary dysentery |
| 14 | Staphylococcus aureus | + | + | Boils carbuncles food poisoning | |
| 15 | Vibrio cholera | + | – | Cholera |
TPCP – Tridax procumbans calyx protein.
Results and Discussion
The TPCP could be purified in good yield on cross-linked guar gum by affinity chromatography8. The purified protein exhibited little low molecular weight of 23kD on SDS – PAGE (Fig. 1 and 2)13. The results show that T. procumbans calyx protein was able to agglutinate pathogenic bacteria. The bacterial agglutination titre was least with Vibrio cholera and Serratia species as compared to other pathogenic bacteria (Table 3).
 |
Figure 1: Electrophoretic mobilities of standard proteinsandT.procumbans calyx protein(TPCP). Acrylamide concentration. 7.5%, standard proteins (a) carbonic anhydrase, (b) – Trypsin and T.procumbans calyx protein (TPCP). (c) Myoglobin, (d) Cytochrome C (from top to bottom) |
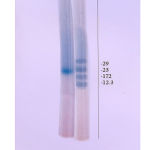 |
Figure 2: SDS-PAGE: (a). T. procumbans calyx protein (TPCP), (b). Standard proteins |
Table 3: Agglutination of pathogenic bacteria with TPCPa.
|
No |
Name of organism and Agglutination with TPCP |
No.of Samples |
Bacterial
|
|||||
| Average titre | Agglutination Units | |||||||
| Crude | ASFb | AC
ASFc |
Crude | ASF | AC
ASF |
|||
| 1 | Enterococci
faecalis + |
5 | 1:2 | 1:4 | 1:8 | 512** | 256** | 128* |
| 2 | Escherichia
coli + |
5 | 1:2 | 1:4 | 1:8 | 512** | 256** | 128* |
| 3 | Klebsiella pneumonie – | 5 | – | – | – | – | – | – |
| 4 | Morganella species +
|
5 | 1:2 | 1:4 | 1:8 | 512** | 256** | 128* |
| 5 | Proteius
mirabilis + |
5 | 1:2 | 1:4 | 1:8 | 512** | 256** | 128* |
| 6 | Pseudomonas
aeruginosa + |
5 | 1:2 | 1:4 | 1:8 | 512** | 256** | 128* |
| 7 | Providentia species + | 5 | 1:2 | 1:4 | 1:8 | 512** | 256** | 128* |
| 8 | Salmonella
typhi – |
5 |
– |
– |
– |
– |
– |
– |
| 9 | Salmonella paratyphi- A – |
5 |
– |
– |
– |
– |
– |
– |
| 10 | Salmonella paratyphi-B – |
5 |
– |
– |
– |
– |
– |
– |
| 11 | Salmonella entritidis – |
5 |
– |
– |
– |
– |
– |
– |
| 12 | Serratia species + | 5 | 1:4 | 1;8 | 1:16 | 256** | 128* | 64** |
| 13 | Shigella
dysenteria + |
5 | 1:2 | 1:4 | 1:8 | 512** | 256 | 128* |
| 14 | Staphylococcus aureus + | 5 | 1:2 | 1:4 | 1:8 | 512** | 256 | 128* |
| 15 | Vibrio cholera + | 5 | 1:4 | 1:8 | 1:16 | 256** | 128* | 64** |
Control
Normal saline
Plant protein only.
*p <= 0.001, **p <=0.02, ***p<=0.05.
TPCP – Tridax procumbans calyx protein.
ASF – Ammonium sulphate fraction.
AC-ASF – Affinity column Ammonium sulphate fraction.
Experiments were run in triplicates
The characteristics of bacterial strains that specifically were agglutinated by TPCP are shown in Table 2 and 3; Fig. 3. TPCP proved to be effective agglutinating agent for Staphylococcus aureus, Enterococci faecalis, Escherichia coli, Pseudomonas species, Shigella species, Proteius mirabilis, Morganella species and Providentia species. Whereas, Salmonella species and Klebsiella species were unable to agglutinate with TPCP. The bacterial strains gave positive agglutination after 4th serial dilution. The strains of Serratia and Vibrio cholera required lowest titre for agglutination as compared to the other bacteria (Table 3; Fig. 3).
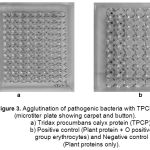 |
Figure 3: Agglutination of pathogenic bacteria with TPCP (microtiter plate showing carpet and button). a) Tridax procumbans calyx protein (TPCP). b) Positive control (Plant protein + O positive group erythrocytes) and Negative control (Plant proteins only). |
The clumped bacteria by the extract of Tridax procumbans L. did not show growth on solid medium (Fig. 4).
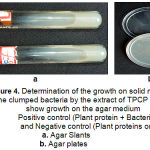 |
Figure 4: Determination of the growth on solid medium: The clumped bacteria by the extract of TPCP did not show growth on the agar medium. Positive control (Plant protein + Bacteria) and Negative control (Plant proteins only). Agar Slants; Agar plates. |
The agglutination of bacteria with TPCP indicates that these bacteria carry the receptor sites. This could be recognized by TPCP and bind with the bacteria thereby forming clumps.
The binding properties and specificities of TPCP may have practical benefit; and it can be used to classify the bacteria, which can serve as an additional test for identification of several pathogenic bacteria. For instance, these studies could be used as a screening test for Salmonella species and Klebsiella that do not have the property to agglutinate with TPCP. In addition, the studies may serve as the basis of a novel therapeutic approach14. Plant proteins are able to selectively clump Pyogenic cocci, similarly, groups A, B, C, F and G streptococci can be readily distinguished. Salmonella genus was unable to react with Con A. The Shigellae are simpler from classification viewpoint because they do not possess flagellar H-antigens and consists of four species. Of the four main Shigella species tested, only Shigella flexneri was readily clumped by Con A. Shigella boydii and Shigella dysenteriae reacted only slowly with the lectin Con A suggesting that it is possible that lectins could classify Enterobacteriaceae, Salmonella genus and Shigella species 8.
The results presented here indicate that the probable specific receptor for TPCP that agglutinated pathogenic bacteria and obstruct their growth by blocking the surface receptors. Thus this extract can be used for the treatment of bacterial dysentery or for preparation of creams for topical application on wounds to prevent bacterial invasion of tissues.
Acknowledgements
The authors thank Dr. Jalgaonkar, Head, Department of Microbiology, IGGMC Nagpur, for providing bacterial strains and grateful to the Head, Department of Biochemistry, R. T. M. Nagpur University, Nagpur for encouragement.
Precautions
Safe working and the prevention of infection was strictly followed according to Health Services Advisory Committee (2003).
References
- Nilsson C., Lectins: Analytical Tools from Nature.; Lectins, Pages 1-13, (2007).
- Persson, M., Wehtje, E. and Adlercreutz, P., Immobilization of lipases by adsorption and deposition: High protein loading gives lower water activity optimum. Lett., 22: 1571-1575, (2000).
- Reetz, M., Zonta, A. and Simpelkamp, J., Efficient immobilization of lipases by entrapment in hydrophobic sol-gel materials. Bioeng., 49: 527-534, (1996).
- Doyle, R. J., Nedjat-Haiem, F. and Doyle, R. J., Clin. Microbiol., 19: 383-387, (1984).
- Cole, H. B., Ezzell, J. W. Jr., Keller, K. F. and Doyle, R. J., Clin. Microbiol., 19: 48 – 53, (1984).
- Prokop, O. and Kohler, W., Agglutinin reactions envon Mikroorganismen mit Helix pomatia Z. Immunitaetsforsch Allerg . Klin. Immunol., 133: 176 -179, (1967).
- Kohler, W. and Prokop, O., Agglutinins versuchean Streptokokken mit dem Phytoagglutinin and Dolichos biflorus. Immunitaetsforsch Allerg . Klin. Immunol., 133: 171-175, (1967).
- Ramteke A., P. and Patil M., B., Purification and Characterization of Tridax procumbans calyx lectins. Biosci. Res. Asia. 3(1), 103 – 110, (2005).
- Hankins, C. N., Kindinger, J. I., and Shannon, L. M., Plant Physiol., 65: 618 – 622, (1980).
- Weber, K., and Osborn, M., Biochem., 244: 4406 – 4412, (1969).
- Davis, B, J., Annals of New York Academy of Sciences., 121: 404 – 427, (1964).
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., Biochem., 193: 265 – 273, (1951) .
- Zanette, D., Sarubbi, E., G., Soffientini, A. and Restelli, E., Process for the Purification of Glycoproteins like Erythropoietin. European Patent Office, EP 0 820 468 B1, (2000).
- Goto, M. Hikota, T., Nakajima, M., Kakikawa, Y. and Tsuyumi, S., Occurance and properties of copper resistance in plant pathogenic bacteria. Annu. Soc. Japan., 60: 147-153, (1994).
- Mulvey, G., Kitov, P., , Marcato, P., Bundle, D., R. and Armstrong, G., D. Glycan mimicry as a basis for novel anti-infective drugs. Biochimie., 83: 841–847, (2001).
- Health Services Advisory Committee; Safety in Health Service Laboratories, Safe working and the prevention of infection in clinical laboratories and similar facilities. 2nd ed. Suffolk: H. S. E., Books; (2003).







