Farideh Sharifi and Latifeh Pourakbar
Department of biology, Urmia University.
Corresponding Author E-mail: f.sharifi 12@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1061
Abstract
Barberry belongs to the Berberidaceaethat It's used in traditional medicine has a long history. The aim of this study was to compare the capacity of nitrite radical gathering, superoxide, Malondialdehyde and power reduction in ethanol, methanol and water extracts of hybrid Berberis Integerrima×Vulgaris. The aqueous extract of fresh barberries the maximum capacity of radical nitrite gathering (723/60±64/56) and the maximum capacity of superoxide radical gathering(202/98±1/75).The ethanol extract of dried barberries the maximum power reduction(0/37±0/006) and The methanol extract of fresh barberries maximum amount of MDA(37/12±0/79 mmol/g), was obtained. The results showed that barberry fruits have high Anti-oxidative capacity which can be used as a plant source in the food and pharmaceutical Industries.
Keywords
Anti-Radical properties; phenolic compounds; Barberry
Download this article as:| Copy the following to cite this article: Sharifi F, Pourakbar L. The Anti-Radical Properties of Phenolic Compounds of Dry and Fresh Barberry (Berberis Integerrima × Vulgaris). Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Sharifi F, Pourakbar L. The Anti-Radical Properties of Phenolic Compounds of Dry and Fresh Barberry (Berberis Integerrima × Vulgaris). Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11111 |
Introduction
Antioxidants by neutralization of free radicals contribute to not only the decrease of cardiovascular disease and heart attacks but also to the prevention of cancer growth .in addition,antioxidants protect the cell membrane(lien et al,2008).Immune system isn’t able to eradicate free radicals by the existence of different antioxidants in the body and for this process needs to get help of the antioxidants in the food.(Halliwall and Gutterige,1998).It’s proved that antioxidants which exist in some nutrients such as butylated Hydroxyanisole,Tert-butyl hydroxyl toluene and beta hydroxyl-Quinone are poisonous and cause malnutrition.So, nowadays, nutritionists advise the consumption of fruit and vegetables in order to provide the necessary antioxidants for the body.The consumption of vegetal antioxidants has lesser side effects and is more beneficial(Franklin,1991).There is a wide range of secondary metabolites in vegetables which protects us against environmental challengeseither alive or dead (Dixon, 2001)Fruit and vegetables have been studied in different countries in order to investigate the existence of phenol and anti-oxidation activity it is proved that the consumption of fruit and vegetables contributes to the eradication of cardiovascular disease , different cancers and senescence .(Mohammad et all 2007; yang et al ,2009; Yu et al ,2005).When the inhibitory mechanism of body which consists of anti-oxidation system fails, free radicals cause cancer, atherosclerosis and other physiologic disorders (Abe et al 2010)Therefore, it is essential to receive anti-oxidative compounds through eating.Among derived antioxidants from vegetables, phenols and flavonoids have strong activity in collecting ROS and decrease the risks of cardiovascular disease. There are phenolic compounds in both food and non-food parts of vegetables and they have a variety of biological effects such as the capacity of free radical gathering(Rozan et al, 2007).
One of the most important destructive effects of free radicals is lipid peroxidation which cause the destruction of cell membrane the oxidation of unsaturated fatty acids leads to the reduction of membrane fluidity and the destruction of its structure and function. So the small amounts of antioxidants in immune system protects cell membrane and different compounds of a creature against oxidants (Rice –Evans and Burdon 1994).Barberry is full of different minerals and vitamins and its nutritional value is high. Barberry’s vitamin C is more than lemon’s(Kafi and Balandri, 1381). Barberry consists of different minerals such as: Tron, calcium , magnesium , sodium , potassium , copper ,zinc, manganese, phosphor , lipid , protein, reducing sugars and anthocyanins.(BerenjiArdestani et al , 2010) . Biological compounds of barberry are used is food and medical industries the usage of barberry as a natural colorants instead of artificial and harmful coloranty has been studied (Sharifi et al 2007). Barberry has been used in different ways for a long time in traditional medicine of Iran (Zargari, 1993; Fatehi –Hassanabad et al, 2008). Berberine is one of this plant’s compounds which can prevent coronary artery disorders and eventually decrease the level of total cholesterol and triglyceride(Kong et al, 2004). Important chemical components of barberry are Berberine, Oxyacanthine and Berbamine. Berberine is the main alkaloid which is separated from barberry’s root and skin. Barberry’s extract consist of different flavonoids like quercetin, Chrysanthemin, Hayprvzyd, delphinidin-3-o-Beta-D-glucoside, Plargvnyn and Petunidin-3-O-Beta-D-Glucoside(Gilgon-Sherki et al , 2001).Besides, it is consists of anti-oxidative compounds like ascorbic acid, alpha –Tocopheroland-beta-carotene(Ferre et al , 2001).Nowadays, because of bad effects of synthetic antioxidants, there is an increasing tendency to the consuming of natural antioxidants . therefore , in this study , the capacity of anti-radicalism of dry and fresh barberry in water, methanol and ethanol extracts has been compared in order to find the best conditions of keeping barberry . Barberry could be used in different areas such as food, medical and pharmaceutical industries.
Methods and materials
Plant gathering
Berberis integerrima*vulgaris was gathered in November 2019 from Qamchoqai zone in Bijar and it’s preserved in freezer till the evaluation time.
Extract preparation
The different extracts of barberry were prepared by maceration. 1g of dry and 2g for frozen barberry was grinded and it was completely squashed and finally it was mixed with 25ml methanol for 2 hours in a shaker. This mixture was passed filters and this extract was kept in special tube away from sunlight in the refrigerator. For preparing water and ethanol extracts, above procedures were repeated.
The capacity of nitrite radical gathering
The amount of Nitrite radicals was determined by GriessIllosvoy reaction(Garrat, 1964). 2 ml Nitroprusside Sodium(10 mmol) and 0/5 ml Phosphate Buffered Salinewas mixed with 20 µl of extract and it was incubated for 150 minutes in the room temperature. After incubation, 1 ml of it was mixed with Sulfanilic Acid (0/33 in glacial Acetic Acid %20) and it was left for 5 minutes. Then, 1ml Naphthyl Ethylene Diamine Hydrochloride 0/1 % was added to it and after 30 minutes the amount of absorption was measured by a Spectrophotometer (at 540 nm). The percentage of radical gathering was calculated by below equation:
Percent of Nitrite radical gathering= (A blank– A sample)*100/A sample
Determining of reduction power
The power of extracts for Iron reduction was measured by Yildirim et al (2001) method with a little change. For measuring the reduction power, 2/5 ml Ferro cyanide and 2/5 ml TCA was added to 100µl extract 2/5 ml Buffer (ph.=6/6). Then, it was put in a Ben Murray for 30 minutes. 0/5 ml FeCl3 was solved in 2 ml distilled water in attest tube. Then, 2 ml of previous solvent was added to this solvent and the amount of absorption was measured at 700 nm. The high amount of absorption, the high amount of reduction power(Oyaizu, 1986).
Determining of Lipid Peroxidation by Thiobarbitoric Acid method:
TBA method was used for the measurement of Lipid Peroxidation. It’s bonded to TBA at 100C and low PH and a red beverage was formed which can be determined at 432 nm. 2ml TCA 20% and 2ml TBA 67% was added to 0/5 ml of extracts. This solution was put in Ben Murray for 20 minutes at 100 C. after that it was reached to room temperature, it was centrifuged for 20 minutes with 3000Rpm. Anti-oxidative activity based on Zincoid solution, absorption is at 532 nm. The amount was determined by below equation:
MDA/GFW= (absorption/ 0/55) * 1000* dilution times
The capacity of Superoxide radical gathering
The amount of superoxide radical gathering was determined by Pyrvgall system and Jing et al (1995) method. 9ml Tris Buffer-Hydrochloric Acid (ph=8/2 and 50 mmol/l) was added to the tube test. This tube was incubated in a warm bath at 25 C for 20 minutes. 40 µl of Pyrvgall solution (45mmol* HCL 10mmol/l) which had been incubated at 25 C before adding to the tube test. This solution was incubated at 25 C for 3 minutes and then one drop of Ascrobic Acid was added to that solution. The absorption at 420 nm after 5 minutes as A0 was measured. The speed of auto oxidation (A1) was measured by Pyrvgall method. This time, certain amount of extract was added to Tris Buffer-HCl solution. Finally, a control factor as A2 was determined. The percentage of superoxide radical gathering was determined by below formula:
The percentage of superoxide radical gathering= A0 – (A1-A2)*100/A0
Statistical analysis
All tests were repeated for three times and results were shown in form of average and standard deviation (SD). The variation between samples was defined and analyzed by bilinear variance (p<%5). Tables and diagrams were drown by SPSS and EXCEL software.
Discussion and results
The capacity of Nitrite radical gathering
According to the results which are shown in diagram 1, fresh barberry in comparison with dry barberry has more capacity of Nitrite oxide gathering. The maximum amount of Nitrite radical was in water extract of fresh barberry(723/60+–64/56+) and the minimum amount of Nitrite radical gathering was in water extract of dry barberry (280/91=-15/41). Nitrite radical gathering activity depends on the density of the extract (Komaran and Karunakaran,2008). NO and NO2 because of having unpaired electron are free radicals. NO is a strong inhibitor in some physiologic procedures such as: controlling blood pressure,relaxing muscles, transferring neural signals, aggregation of platelets and having antibacterial and anticancer properties.(Hageman et al,1998). It is proved that plants use NO as a growth regulator which moderates anti-bacterial reactions(Sakamoto et al,2002). Recently, it’s proved that this substance plays a vital and important role in the regulation of normal physiologic activities of plants like stomatal closure, growth and evolvement(Zhua et al,2006).
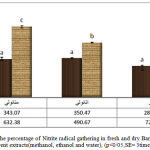 |
Figure 1: the percentage of Nitrite radical gathering in fresh and dry Barberry in different extracts(methanol, ethanol and water), (p<0/05,SE= 3times) |
In Jiao et al(2008) and Nikkhah et al(2009) studies, Phenolic compounds are good sources of antioxidants. The more extract’s dose is, the more scavenging percent of phenolic compounds.It’s shown in Sasikumar et al (2012) studies thatdifferent densities of barberry’s extracts have a good inhibitory potential in comparison with Nitric oxide radical. In this study, the results are the same.
Determining of reduction power
The reduction power of methanol, ethanol and water extracts of fresh and dry barberry has been measured by transformation of FE3+ to Fe2+ (diagram 2).There wasn’t any difference in reduction power of different extracts(absorption at700nm). The maximum amount of reduction power was in the ethanol extract of dry barberry(0/371+-0/006|) and the minimum amount of reduction power was in water extract of fresh barberry(0/294+-0/009). The iron reduction method is the fastest and the best way for measuring the reduction power of chemical compounds and it can be used as an index of antioxidant power of chemical compounds(Gulcin,2009). Antioxidants with a high reduction power of iron have a good ability in ending the destructive chain reactions of free radicals’ formation (Wanasundara and Shahidi, 2005). In this method, reduction power of nutrients in the sample is measured by reducing Fe3+ to Fe2+ (Chung et al, 2006). The reduction of iron 3 is mainly used as the index of electron activity (Hinneburg et al, 2006). Different extracts of barberry have a high reduction power (Mohammadi et al, 2012). The reduction power of methanol extract is less than BHT (Sasikumar et al, 2012). The potential of reduction power of fresh barberry is more than the dry barberry (Beyhan et al, 2010). The results showed that the reduction power of barberry extract is because of the polyphenol’s presence that these polyphenols act as a reduction.
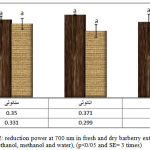 |
Figure 2: reduction power at 700 nm in fresh and dry barberry extracts (ethanol, methanol and water), (p<0/05 and SE= 3 times) |
Determining of Lipid Peroxidation by Thiobarbitoric Acid method:
The measurement of MDA in methanol, ethanol and water extracts of dry and fresh barberry is shown in diagram 3. The maximum amount of MDA was in methanol extract of fresh barberry (378/12+-0/79) and the minimum amount of MDA was in water extract of dry barberry (8/24+-0/6). In both dry and fresh barberries, methanol and water extracts have the maximum and minimum amounts of MDA. The amount of Lipid Peroxidation in tissues was measured by Thiobarbitoric Acid method. When oxidative tension occurs, Lipid Peroxidation of unsaturated fatty acids increases. Because of the free radicals’ attack to Lipids, different Aldeids such as MDA will be created (Mates et al, 1999). The production of Lipid Peroxidation with a high and free radical is a sign of a toxic condition and its final product is MDA. The increased MDA is an index for physiologic tension (Pourakbar et al, 2007). This anti-oxidative effect is because of the phenolic extracts. As a matter of fact, by increasing the density, the amount of phenolic compounds increases and this causes the inhibitory of free radicals.
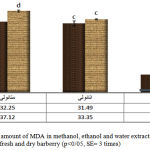 |
Figure 3: the amount of MDA in methanol, ethanol and water extracts of fresh and dry barberry (p<0/05, SE= 3 times) |
These compounds because of having Hydroxyl group have the ability of free radicals’ neutralization. By adding the skin and the seed of grape’s extract to the sunflower oil, Emad(2006) understood that the anti-oxidative power of the grape’s skin is more than its seed. In other words, phenolic compounds by cleansing free radicals prevent from Lipid Oxidation and the MDA increases. In total, barberry’s extract as a source of natural antioxidants has the ability to react against free radicals. In this study, the amount of MDA had a reverse ratio with the amount of total phenol in different extracts of the barberry. i.e. methanol extracts had the maximum and water extracts had the minimum amount of MDA.
The capacity of superoxide radical gathering:
For measuring the capacity of superoxide radical gathering, different extracts of fresh and dry barberry were studied by Pyrvgall method. According to the given data in diagram 4, the maximum amount of SO was in water extract of fresh barberry (202/98-+1/75) and the minimum amount of SO was in methanol extract of dry barberry (134/54+-0/59). SO radical is one of the most dangerous free radicals in human body (Schlesier et al, 2002). So radical is so toxic that it is a sub-product of Mitochondrial respiration. The capacity of SO radical gathering was studied by Pyrvgall oxidation system. The capacity of SO radical gathering in different densities of methanol extract of barberry has been studied in comparison with BHT. The amount of radical gathering in barberry’s extract was significantly more than the BHT’s synthetic antioxidant. By increasing the sample’s density, the reducing power of SO radical increases (Jiao et al, 2005). In this study, the anti-radical activity of different extracts (specially the fresh barberry’s extract) was good.
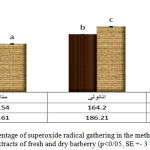 |
Figure 4: The percentage of superoxide radical gathering in the methanol, ethanol and water extracts of fresh and dry barberry (p<0/05, SE +- 3 times) |
Conclusion
In different studies, it is proved that phenolic compounds like flavonoids have the anti-oxidative and anti-radical effects such as: the capacity of Nitrite oxide radical gathering, superoxide and anti-oxidative activities. It is concluded that the fresh barberry is rich of phenolic compounds and it can be used in both food and pharmaceutical industries. The amount of nitrite radical gathering, MDA and SO radical gathering in fresh barberry was more than in the dry barberry, but there was no significant difference in reducing power. In other words, we recommend the use of both fresh and dry barberry. Anyway, both fresh and dry barberry have the anti-oxidative activity and are introduced as a source of phenolic compounds. Based on the results, different extracts have a high nutritional value, but the use of water extract is highly recommended because it is harmless.
References
- Abe,L.T., Lajolo, F.M. and Genovese, M.I. 2010. Comparison of phenol content and antioxidant capacity of nuts. CienciaeTechnologia de alimentos. 30: 254-259.
- Asada, K. 2000. The water-water cycle as alternative photon and electron sinks. Philosophical Transactions of the Royal Society. 355:1419-1431.
- BerenjiArdestani, S., Sahari, M. A., Barzegar, M. and Abbasi, S. 2013. Some physicochemical properties of Iranian native barberry fruits (abi and poloei): Berberisintegerrimaand Berberis vulgaris. Journal of food and Pharmaceutical Sciences.1: 67-74.
- Beyhan, Ö., Elmastaş, M. and Gedikli, F. 2010. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Accasellowiana, Myrtaceae). Journal of Medicinal Plants Research. 4(11): 1065-1072.
- Chung, Y.C., Chien, C.T., Teng, K.Y. and Chou, S.T. 2006. Antioxidative and mutagenic properties of Zanthoxylumailanthoides Sieb & zucc. Food Chemistry. 97: 418- 425.
- Davey, M.W. E., Stals, B., Panis, J., Keulemans, R. L. Swennen. 2005. High Throughput determination of malondialdehyde in plant tissues. Analytical Biochemistry. 347: 201-207.
- Dixon, R.A. 2001. Natural products and disease resistance. Nature. 411: 843–847.
- Emad, S. 2006. Antioxidant effect of extracts from red grape seed and peel on lipid oxidation in oils of sunflower. LWT Food Science and Technology. 39: 883-89.
- Fallahi, J., RezvaniMoghaddam, P. and NasiriMahallati, M. 2010. Effects of harvesting time quantitative and qualitative properties of Berberis vulgaris fruit. International Journal of Field Crops Research(In Farsi). 8 (2): 225-2.
- Fatehi-Hassanabad, Z., Jafarzadeh, M., Tarhini, A. and Fatehi, M. 2005. The anti hypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive. Phytotherapy Research. 19: 222-225.
- Ferre, N., Camps, K., Cabre, M., Paul, A. and Joven, J. 2001. Hepatic cirrhosis. paraoxygenase activity alterations and free radical production in rats with experimental. Metabolism. 50(9): 997-1000.
- Frankel EN. 1991. Recent advances in lipid oxidation. Journal Science Food Agricultural. 54: 495-511.
- Garrat, D.C. 1964. The quantitative analysis of drugs. Chapman and Hall 1th. Japan. 3: 456-458.
- Gilgun-Sherki, Y., Melamed, E. and Offen, D. 2001. Oxidative stress induced neuro-dengenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 40 (8): 959-975.
- Gülçin , I. 2009. Antioxidant activity of L-adernaline: A structure-activity insight. Chemico-Biologycal Interaction. 179: 71-80.
- Hagerman, A.E., Riedl, K.M., Jones, G.A., Sovik, K.N., Ritchard, N.T. and Hartzfeld, P.W. 1998. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry. 46: 1887-1892.
- Halliwell B, Gutteridge JMC. 1998. Free radicals in biology and medicine. 4th ed. Oxford, UK: Clarendon Press. 617-78.
- Hinneburg, I., Damien Dorman, H.J. and Hiltunen, R. 2006. Antioxidant activities of selected culinary herbs and spices. Food Chemistry. 97: 122-129.
- Jiao, Z.H., Lio, J. and Wang, S. 2005. Antioxidant activity of total pigment extract from blackberries. Food Technology and Biotechnology. 43: 97-102.
- Jing, T.Y. and Zhao, X.Y. 1995. The improved pyrogallol method by using terminating agent for superoxide dismutase measurement. Progress Biochemistry and biophysics. 22:84-86.
- Kong, W., Wei, J., Abidi, P., Lin, M., Inaba, S. and Li, C. 2004. Berberine is a novel cholesterol lowering drug working through a unique mechanism distinct from statins. Nature Medicine . 10:1344- 1351.
- Kumaran, A. and Karunakaran, R.J. 2006. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chemistry. 97: 109-114.
- Lien Ai PH, Hua H, Chuong PH. 2008. Free Radicals, Antioxidants in Disease and Health. International Journal of Biomedical Science. 4(2):89-96.
- Mates, J.M., Perez-Gomez, C. and Nunez de Castro, I. 1999. Antioxidant enzymes and human diseases. Clinical Biochemistry. 32(8): 595 – 603.
- Mohamadi, M.A., Maskooki, M. and Mortazavi, S. A. 2012. Evaluation of antioxidant properties of barberry fruits extracts using maceration and subcritical water extraction. World Academy of Science, Engineering and Technology. 69: 344-348.
- Mohamed, R., Pineda, M. and Aguilar, M. 2007. Antioxidant capacity of extracts from wild and crop plants of mediterranean region, sensory and nutritive qualities of food. Journal of Food Science. 72: 59-63.
- Nikkhah, E., Khayami, M. and Heidary, R. 2009. In vitro antioxidant activity of berry (Morusalba var.nigra). International Journal of plant Production. 3: 15-18.
- Oyaizu, M. 1986. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucoseamine. Japanese Journal of Nutrition. 44:307-315.
- Pourakbar, L., Khayami, M., Khara J. and Farbodnia, T. 2007. Copper-Induse change in antioxidative system in maize (Zea mays L.). Pakistan Journal of Biological Sciences. 10:3662-3667.
- Rozan, P., Hidalog, S., Nejdi, A., Bisson, J.F., Lalonde, R., and Messaoudi, M. 2007. Preventive antioxidants effects of cocoa polyphenolic extract on free radical Production and cognitive performances heat exposure in wistar rats. Journal of Food Science. 72: 203-206.
- Rice-Evans, C.A. and Burdon, R.M. 1994. Free radical damage and its control. Amsterdam Elseri. 113: 46-49.
- Sakamotoa, A., Uedab, M. and Morikawa, H. 2002. Arabidopsis glutathione dependent formaldehyde dehydrogenase is an S-nitros glutathione reductase. FEBS Letters. 515: 20-24.
- Sasikumar, J. M., Maheshu, V., Smilin, A. G., Gincy, M. M. and Joji, C. 2012. Antioxidant and antihemolytic activities of common Nilgiri barberry (BerberistinctoriaLesch.) from south India. International Food Research Journal. 19 (4): 1601-1607.
- Schlesier, K., Harwat, M., Böhm, V. and Bitsch, R. 2002. Assessment of antioxidant activity by using different in vitro methods. Free Radical Research. 36: 177-187.
- Sharifi, A., Tavakolipour, H., Maskooki, A. and Elhamirad, A. H. 2008. Evaluation of barberry colour extraction. 18th National Congress on Food Technology. Mashhad, I. R. Iran (In Farsi). 268.
- Wanasundara, P. K. and shahidi, F. 2005. Antioxidants: science, technology, and pplications. In bailey,sindustrial oil and fat products. Shahidi F. (Eds). John Wiley and Sons, Inc. New Jersey.
- Yang, J., Lio, R. and Halim, L. 2009. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Science and Technology. 42: 1-8.
- Yildirim, A., Mavi, A. and Kara, A.A. 2001. Determination of antioxidant and antimicrobial activities of RumexcrispusL. extracts. Journal of Agricultural and Food Chemistry. 49: 4083-4089.
- Yu, J., Ahmedna, M. and Goktepe, I. 2005. Effects of processing methods an extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chemistry. 90: 199-206.
- Zargari, A., ed. 1993. Medicinal plants. 7th ed. Tehran: Tehran University Press. 72-79.
- Zhua, S., Liu, M. and Zhou, J. 2006. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biology and Technology. 42:41–48.








