Manuscript accepted on :December 17, 2016
Published online on: --
Plagiarism Check: Yes
Maryam Akbari1*, Alireza Almasi2 , Zahra Naderi3 , Jalil Kouhpayezadeh4 , Roghaye Pourali1 and Zohreh Hossinzadeh5
1Shahid Akbar Abadi Hospital, Iran University of Medical Sciences, Tehran, Iran.
2Department of Radiology, Firouzgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
3Department of Midwifery and Gynecology, Shahid Akbar Abadi Hospital, Iran.
4Department of Social Medicine, Medicine College, Iran University of Medical Sciences, Tehran, Iran.
5Neonatal Intensive Care Nursing, Tehran University of Medical Sciences , Tehran, Iran.
*Corresponding Author E-mail: maryir85@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1046
Abstract
Various researches have been conducted over the recent years on the therapeutic effects of statins on the metabolic and hyper-androgenic state of the patients suffering from PCOS. The present research seeks to evaluate the treatment with atorvastatin and its effect on the lipid profile level, serum androgen status and morphology and blood flow of polycystic ovaries. A double blind clinical trial was designed for this research where the women with PCOS resorting to the gynecology clinic of Firouzgar Hospital were randomly divided into two groups: case and Control . Early at the beginning of the research, variables such as body mass, lipid profile, blood androgen level, fasting blood Sugar , size of the ovary, and resistance of the stromal artery of ovary were studied. For a period of 6 weeks, one group was given with a daily dose of 40 mg atorvastatin, while the other group just received placebo. All the variables were studied once again after 6 weeks and the results were analyzed using SPSS v.16. The case group included 20 patients suffering from PCOS who received atorvastatin, while there were 20 patients with PCOS in the witness group who just received placebo. The average ages in the atorvastatin and placebo groups were 27.7 ± 3.41 and 9.30 ± 4.8 years old respectively. A significant difference was observed between the two groups in terms of changes in the average cholesterol and LDL levels before and after the intervention. This reduction was more significant in the atorvastatin group. After prescription of atorvastatin, the level of Androstenedione had decreased significantly in treatment group. A statistically significant reduction was observed in the size of left and right ovaries in the group receiving atorvastatin. No significant changes were observed in the size of the ovaries in the group receiving placebo. The average arterial resistance level of left and right ovaries before and after intervention in atorvastatin group exhibited a significant reduction. Having discarded the confounding effect of RI, this difference with the witness group was statistically significant. Keeping in mind the effects of atorvastatin such as improving the lipid profile status and reduction of androstenedione among those with PCOS, it can be used as an auxiliary treatment to control symptoms and long-term side effects among patients. Considering the shrinkage of ovary size and enhancement of blood flow to PCOS ovary, future researches can focus on effectiveness of statins in improving the ovulation status of performance of PCO ovaries.
Keywords
Hyper-androgenism, Arterial Performance of Ovary, Hyperinsulinemia, Atorvastatin. PCOS
Download this article as:| Copy the following to cite this article: Akbari M, Almasi A, Naderi Z, Kouhpayezadeh J, Pourali R, Hossinzadeh Z. The Effect of Atrovastatin on the Ovarian Arterial Blood Flow and Serum Androgen Level in PCOS Patient. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Akbari M, Almasi A, Naderi Z, Kouhpayezadeh J, Pourali R, Hossinzadeh Z. The Effect of Atrovastatin on the Ovarian Arterial Blood Flow and Serum Androgen Level in PCOS Patient. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11756 |
Introduction
Polycystic ovary syndrome is a syndrome with various symptoms and signs. It is also the most common endocrine pathology among women observed during the ages of fertility, but it is far from being exclusive and hence no particular cause can be introduced for it (1). Various definintoin have been proposed for this syndrome. According to Rotterdam criteria, the presence of 2 of these 3 symptoms indicates this syndrome: 1. no or little ovulation (Oligomenorrhea or Amenorrhea), 2. hyper-androgenomia or hyper-androgenism, and 3. polycystic ovaries (diagnosed through sonography and through discarding other complications with too much discharge of androgen or correlating complications) (1). Nearly 70% of polycystic ovary syndromes exhibit signs of the abnormal lipid levels in body (2). Women with PCOS whether fat or not exhibit degrees of Dyslipidemia and these changes are observed in all three levels of TG, HDL, and LDL (3). As various researches have shown, further to reducing the lipid level of blood, atorvastatin also helps decrease androgen (4). In this syndrome, there is a direct correlation between levels of androgen and insulin resistance and blood lipids level. Reducing the lipid level of blood can help improve the androgen level and enhance Hyperinsulinemia and reduce the side effects of this disease (4). Various researches have also pointed to the fact that atorvastatin can help improve the blood flow of Brachial artery (1). Although no studies have ever been conducted on this medicine and blood flow of ovary artery, we have set to study the effect of this medicine on ovary artery given the available literature indicating improvement of DOMINANT ovary and ovulation with the rise and enhancement of blood flow of ovary artery (5). Considering the problems of those with this disease (obesity, hyperinsulinemia, hyper-androgenomia, no ovulation and Hirsutism), it is necessary to seek an appropriate cure to this disease and try to enhance the life quality of these patients.
Material and Method
This is an analytical, double blind, clinical trial research. The population includes all the women with PCOD resorting to the clinic of Firouzgar Hospital from November 2004 to March 2005. The patients and the measurers had no idea of the variables associated with attribution of the patients. The following inclusion criteria were defined: 1) diagnosis of PCOS based upon existence of 2 of the 3 criteria specified by Rotterdam, 2) BMI > 25.2, 3) LDL > 100, 4) no pregnancy, 5) no other reasons for obesity and metabolic and hormone and glands and metabolism issues, 6) no use of Metformin and other medicines that create sensitivity to insulin and no use of OCP over the last three months and no consumption of medicines that reduce androgen, 7) no sensitivity to atorvastatin, and 8) aging 18 to 35 years old. Certain exclusion criteria were also defined such as 1) pregnancy, 2) using metformin or any other medicines that cause sensitivity to insulin and OCP over the last 3 months before the research and androgen reducer (using progesterone for withdrawal or keeping mensturation was ok), 3) LDL < 100, 4) BMI < 25, 5) any hormonal or metabolic disorders not related to PCOS, 6) any sensitivity to atorvastatin exhibited by the rise of liver enzymes more than three times the normal level and severe cramps of muscles. People qualified for the research were randomly divided into randomized blocks containing 4 people in each one. Early at the beginning of the research, variables such as body mass, lipid profile, blood androgen level, fasting blood Sugar, size of the ovary, and resistance of the stromal artery of ovary were studied. For a period of 6 weeks, one group was injected with a daily dose of 40 mg atorvastatin, while the other group just received placebo. All the variables were studied once again after 6 weeks and the results were analyzed using SPSS v.16.
The tool to collect data was a questionnaire asking for information such as age, LIPID PROFILE, BMI, the androgen level of blood, size of ovary, levels of insulin, fasting blood Sugar, and resistance of the stromal artery of ovary. All laboratory procedures were carried out in laboratory of Firouzgar Hospital. Only the procedures associated with Androstenedione hormone were undertaken in Nour pathobiology laboratory. You will find the kits and experiment methods and techniques used below:
FBS: (Kit: Biosystem) (Experiment Method: Glu-oxidase) (Technique: Auto analyzer sapphire 800)
Chol: (Kit: Biosystem) (Experiment Method: Chol-oxidase) (Technique: Auto analyzer sapphire 800)
TG: (Kit: Biosystem) (Experiment Method: Peroxidase) (Technique: Auto analyzer sapphire 800)
HDL: (Kit: Biosystem) (Experiment Method: Direct) (Technique: Auto analyzer sapphire 800)
LDL: No kit and through calculation
Testosterone: By ECL machine built by Rosh German Company
DHEAS: By Liason machine by Rosh German Company
Androstenedione: (Diametra kit) (Experiment Method: Eliza)
Vaginal Doppler and sonography were carried out by radiologist in sonography ward of Firouzgar Hospital. A Voluson 730 Expert machine with a 6 to 12 MHz micro convex vaginal probe was used for sonography. 40 mg atorvastatin pills (in the form of calcium tri-hydrate) built by Sobhan Darou Company registered under code 1228058660 (IRC) was used in the case group while 100 mg pills of B1 vitamin produced by Darou Pakhsh Co. was used as placebo for witness group (thiamin hydrochloride). Sequential nonrandom sampling method was used in both groups. The resulting information was analyzed using SPSS 16. As for qualitative variables, indicators such as frequency, mode, mean, and average were calculated. The frequency of qualitative variables was also measured. Independent t test was used to study qualitative variables, while repeated measures test was used to calculate the changes observed in two groups before and after intervention. Forward linear regression was used to study the confounding effect. The ethics committee of Iran University of Medical Sciences studied the ethical aspects of this research and approved it under the code 93/D/105/4431. The patients were not charged for experiments. The results were confidential and only the general results and conclusions were reported. Throughout the stages of this research, the ethical principles specified in treaty of Helsinki were observed. The general results were published in credible scientific resources in order to fulfill the goals. The written consent of the patients was obtained before their entrance to the research.
Results
The present research was conducted to study the effect of atorvastatin on the arterial performance of ovary and androgen level of blood among those suffering from polycystic ovary syndrome. The case group included 20 patients suffering from PCOS who received atorvastatin, while there were 20 patients with PCOS in the witness group who just received placebo. The average ages in the atorvastatin and placebo groups were 7.27 ± 3.41 (22 to 35) and 9.30 ± 4.8 (18 to 35) years old respectively. Table 1 presents the results of laboratory profile for all groups before and after treatment in details.
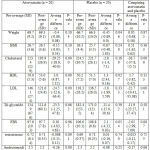 |
Table 1: Metabolic and fat profile and level of androgen hormones in both groups before and after treatment |
The results of sonography for both groups before and after treatment are presented in table 2.
Table 2: Sonography results
| Atorvastatin (n = 20) | Placebo (n = 20) | Comparing atorvastatin and placebo | ||||||||
| Pre-average (SD) | Post-average (SD) | Average difference
(95% CI) |
P-value | Pre-average (SD) | Post-average (SD) | Average difference
(95% CI) |
P-value | Average difference
(95% CI) |
P- value | |
| DHEAS | 225.3
(119.6) |
233.8
(70.5) |
8.45
(-56.0, 39.1) |
0.714 | 232.6
(103.0) |
235.6
(104.4) |
3.02
(-8.91, 2.86) |
0.295 | 5.42 (-51.7, 40.9) | 0.814 |
| Left ovary size | 14.9
(4.9) |
14.2
(4.5) |
-0.75
(-0.05, 1.57) |
0.047 | 13.1
(5.2) |
13.7
(4.2) |
0.58
(-1.48, 0.30) |
0.185 | -1.34
(-2.51, -0.17) |
0.025 |
| Right ovary size | 15.1
(6.3) |
14.2
(6.5) |
-0.92
(-1.76, -0.09) |
0.031 | 13.6
(3.9) |
13.8
(3.9) |
0.15
(-0.54, 0.23) |
0.425 | -1.07
(-1.97, -0.18) |
0.019 |
| Arterial resistance of left ovary | 0.51
(0.07) |
0.48
(0.06) |
-0.03
(-0.05, -0.01) |
0.001 | 0.64
(0.2) |
0.56
(0.1) |
-0.08
(-0.02, 0.18) |
0.126 | 0.04
(-0.15, 0.05) |
0.040 |
| Arterial resistance of right ovary | 0.51
(0.07) |
0.49
(0.06) |
-0.02
(-0.0, 0.04) |
0.051 | 0.58
(0.1) |
0.57
(0.1) |
-0.01
(-0.0, 0.02) |
0.121 | -0.01
(-0.01, 0.03) |
0.037 |
Discussion
Polycystic ovary syndrome is a kind of syndrome with various signs and symptoms and it is far from being an exclusive endocrine pathology, thus it has no exclusive cause. Keeping in mind the frequency and prevalence of this disease and the problems caused by it in terms of hormone, appearance, metabolism and other systematic issues, it will turn into a major infertility problem among those women in the age of pregnancy and fertility. There are several researches and studies conducted on this issue with similar or dissimilar results in terms of the type of research and the population studied. Based on our researches, our paper is the first one to study the influence of atorvastatin on the stromal artery of ovary. Of course, similar studies using metformin have been conducted over this issue (6). Positive effects of statins in reducing arthrosclerosis and cardiovascular diseases have been proved. As a result, statins are used to treat dyslipidemia and to prevent atherosclerosis and cardiovascular issues (7). The present research also studied the possible effect of atorvastatins in reducing the sclerosis of stromal artery of ovary.
A study was conducted by Raja-Khan et al (2011) in order to determine the effects of statins on vascular performance, inflammation and blood level of androgen among women suffering from polycystic ovary syndrome. Of androgen hormones, androstenedione exhibited a significant reduction compared to atorvastatin and placebo (4). However, no significant difference was observed between the two groups in our research. Androstenedione had decreased significantly in the group where participants had received atorvastatin. Hyper-androgenomia is a risk factor that increases the risk of affliction with cardio-vascular diseases and high blood pressure among women with polycystic ovary syndrome (8). These are the Pleiotropic effect of statin: improving the level of CRP, endothelial performance disruption, higher bioavailability of nitric oxide, antioxidant properties, harnessing inflammatory responses, and stability of Atherosclerotic plaques (9). The level of DHEAS serum and testosterone hormone among those suffering from PCOS has been reported to be significantly higher than what was observed among healthy women (10). Sathyapalan et al (2012) conducted a research in order to see the effects of atorvastatin on density of testosterone, androstenedione, DHEA, and DHEAS among those suffering from PCOS. The results reported a significant reduction of androstenedione and DHEAS among those patients who had received atorvastatin for 3 months. However, no significant changes were observed using the placebo (11). It is important to keep in mind that as the length of treatment becomes longer, it is better to use a lower dose of this medicine and this suggestion was followed and observed in other researches conducted so far. Puurunen et al (2013) studied the effects of atorvastatin treatment on hormone parameters and metabolism of women suffering from PCOS (12). They reported a reduced level of DHEAS serum in atorvastatin group while no change was observed in the testosterone level of serum. Meanwhile, the results of our research indicated no decrease of these 2 parameters in any of those two groups. Gao L et al (13) conducted a meta-analysis and reported a significant difference in total reduction of testosterone serum while comparing statin with placebo. This is quite the opposite to the results we achieved in our research. In another RCT research conducted on 248 women suffering from PCOS in statin and placebo groups, a reduction in the level of testosterone was observed (8).
According to the results of our research, the changes in the average level of cholesterol and LDL in both groups before and after intervention were statistically significant and a higher level of reduction was observed in atorvastatin group. Gao L et al (13) also believe that statin is more effective than placebo in reducing the levels of LDL, TC and TG. In the research conducted by Raval et al (8), statins helped improve the status of total cholesterol, LDL, and TG, but they had no significant influence on HDL, HS, and CRP. These results are so close to the results we achieved. In our research, the HDL level of patients before and after intervention in both the placebo and atorvastatin group exhibited a significant increase. However, no significant difference was observed between the two groups before and after intervention in terms of changes in the level of HDL. Changes in the level of TG were not noticeable in any of these groups.
It was shown in another research that using atorvastatin for 6 months helped reduce the level of FBS and resistance against insulin (12). Using atorvastatin for 40 days in our research showed no significant reduction in the level of fasting blood Sugar. On the other hand, a significant reduction in the level of fasting blood Sugar was observed following treatment with atorvastatine in the research conducted by Mobseri et al (14).
The other two variables studied in this research were size of ovary and resistance of ovary artery. An increased size of ovary is one of the main criteria used to diagnose polycystic ovary syndrome (15). As for the characteristics of this syndrome in sonography, one may refer to an ovary size above 10 cc and observation of 12 or more Follicles in each ovary with a size of 2 to 9 mm and stroma with increased central echo (16). In our research, the size of left and right ovaries exhibited a statistically significant reduction in the group using atorvastatin. However, no significant decrease in the size of the ovaries was observed in the group which had received placebo. All in all, the changes in the size of the ovaries in case group was significantly more than what was observed in witness group which resulted in a statistically significant size reduction. A research conducted in 2011 found no reduction in the size of ovary in any of the two groups. A comparison of the average changes in two groups reported no statistically significant reduction in the size of the ovaries. Keeping in mind the results of the current research and the other research conducted in Pennsylvania, measurement of size changes in larger samples seems useful for recording more effective results (4). No research on those suffering from PCOD was found concerning the indexes of Doppler sonography of stromal artery of ovary. A research was conducted on 41 women with PCOD where posterior cilliary artery, central retinal artery, and orbital artery were studied and compared against control group. Factors such as systolic and diastolic pace had increased in orbital artery and posterior cilliary artery, but arterial resistance index exhibited some kind of reduction. They claimed a relationship between the blood flow speed and levels of cholesterol and a revers relationship between the former and insulin and glucose level of serum (17). Although these researches did not deal with ovary artery, the correlation between Doppler indexes and systemic metabolic state points to the fact that the very same study can be conducted for ovary artery. In this research, a resistive index was taken from both the case and witness groups and all of them were above 0.40 which practically discarded any possibility of malignant masses in ovaries as it has been observed that tumoral arteries have no muscular layer and they are of low resistance (18). Only RI was measured in our research. In another RCT research, FMD (follow mediated dilation) in brachial artery was measured. This value in internal medicine is indicative of long-term cardiovascular incidents. The results pointed to the fact that this case caused no statistically significant change in any one of the two groups. They claimed that the average sum of FMD following consumption of atorvastatin has improved, hence atorvastatin is a useful medicine for these cardiovascular incidents (4). However, RI of stromal artery of ovary exhibited a decrease in the mean on both sides. In statistical terms, the resistance of right stromal artery exhibited a borderline reduction. A statistically significant difference was observed between the two groups in terms of stromal arterial resistance of ovary having eliminated the confounding influence of RI variable. Considering the statistically significant reduction in the average arterial resistance in case group after receiving atorvastatin, this medicine seems really helpful in fulfilling this goal. It is better to study application of this medicine over a longer course of time and in a larger population. Concerning the Doppler indexes of the stromal artery of ovary and consumption of metformin among 25 women suffering from PCOS, RCT study was carried out where they received a daily dose of 850 mg of this medicine for 6 months which resulted in the higher levels of RI and PI (6).
Another method used to treat infertility among those suffering from this syndrome is using Diathermy or cauterization of the surface of ovary. A research conducted on 52 patients 6 weeks after laparoscopy and cauterization on Doppler indexes of stromal artery of ovary demonstrated a greater pace of flow (19). Using a non-invasive medical treatment and without increasing the risk of surgical side effects and early failure of ovary, favorable therapeutic results may be achieved for a patient using a method with good tolerance and little cost.
Research limitations
The small population of the patients due to the strict exclusion criteria (using metformin within the last three months before the research, OCP, any anti-androgen medicines, cardio-vascular diseases, gland and metabolism issues) was the main limitation in this research. This problem can be easily resolved by increasing the number of participants.
Conclusion
All in all, we may conclude that atorvastatin is a relatively useful medicine as it improves some biochemical factors associated with metabolic syndrome of these patients and its favorable consequences on the artery of ovary can not be denied. It is recommended to measure all Doppler indexes on stromal artery of ovary so that more accurate results can be achieved. Based on the results achieved in our research, it is recommended to carry out a study with a larger population where indexes of stromal artery in patients suffering from polycystic syndrome who are undergoing treatment with atorvastatin can be studied in greater details and the results can be used to treat the patients. Keeping in mind the improvement of lipid profile status and reduction of androstenedione levels among those suffering from PCOS, atorvastatin can be used as an auxiliary treatment to control the symptoms and long-terms complications. Concerning the reduction of the size of ovary and improvement in blood flow of PCOS ovary, future researches can focus on the effectiveness of statins in improving the ovulation status and performance of PCO ovaries.
References
- Marc A, Fritz, Leon Sperooff. Clinical genecologyic endocrinology and infertility. Eight Edition. 2011;1(12):498-531.
- E Diamanti-Kandarakis et al. Pathophysiology and Types of Dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18(7): 280-285.
- Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607-13.
- Nazia Raja-Khan ,M.D.Allen R.Kunselman ,M.A.Cynthia S.Hogeman ,R.N.Richard S .Legro ,M.D.Effect of Atorvastatin on vascular function,inflammation and androgens in woman with polycystic ovary syndrome:a double blind ,randomized ,placebo-control trial. Fertil Steril. 2011; 95(5): 1849-52.
- Costello MF1, Shrestha SM, Sjoblom P, McNally G, Bennett MJ, Steigrad SJ, Hughes GJ. .power Doppler ultrasound assessment of ovarian blood flow in women with polycystic ovaries and normal ovary during in vitro fertilization. Fertil Steril. 2005; 83(4): 945-54.
- Ozcimen EE, Uckuyu A, Ciftci FC, Zeyneloglu HB. The effect of metformin treatment on ovarian stromal blood flow in women with polycystic ovary syndrome. Arch Gynecol Obstet.2009; 280(2): 263-9.
- Sohrabvand F, Lankarani M, Golestan B, Javidi E. Serum homocysteine levels in PCOS patients versus healthy women.J Reprod Infertil. 2009; 9(4): 334-341.
- Raval AD, Hunter T, Stuckey B, Hart RJ. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011; 5(10): CD008565
- Parlakgumus HA, Aka Bolat F, Bulgan Kilicdag E, Simsek E, Parlakgumus A. Atorvastatin for ovarian torsion: effects on follicle counts, AMH, and VEGF expression. Eur J Obstet Gynecol Reprod Biol. 2014; 175: 186-90.
- Kadaman PH, Duleba AJ. Statin in treatment of polycystic ovary syndrome. Semin Reprod Med. 2008; 26: 127-138.
- Sthyapalan T, Smith KA, Coady AM.Atorvastatin therapy decrease and rostendione and dehydroepiandrostendione sulphate concentration in patients with polycystic ovary syndrome randomized clinical trial. Ann Clin Biochem. 2012; 49: 80–85.
- Johanna Puurunen, Terhi Piltonen, Katri Puukka, Aimo Ruokonen, Markku J. Savolainen, Risto Bloigu, Laure Morin-Papunen, and Juha S. Tapanainen. Statin Therapy Worsens Insulin Sensitivity in Women With Polycystic Ovary Syndrome (PCOS): A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Endocrinol Metab. 2013; 98(12): 4798-807.
- Gao L, Zhao FL, Li SC. Statin is a reasonable treatment option for patients with Polycystic Ovary Syndrome: a meta-analysis of randomized controlled trials. Exp Clin Endocrinol Diabetes. 2012; 120(6): 367-75.
- Majid Mobasseri, Jafar Shadi, Amir Bahrami, Akbar Aliasgarzadeh, Esmaeil Faraji, Morteza Gojazadeh. A comparison of effects of Atorvastatin and OCP on biochemical profile of PCOS patients. Journal of American Science. 2013; 9(7s).
- Thozhukat Sathyapalan, Eric S. Kilpatrick, Anne-Marie Coady, and Stephen L. Atkin. The Effect of Atorvastatin in Patients with Polycystic Ovary Syndrome: A Randomized Double-Blind Placebo-Controlled Study. J Clin Endocrinol Metab. 2009; 94(1): 103-8.
- Anna S, Lev Toaff , Deborah Levine John P, McGahan Barry B Goldberg. Diagnostic sonography female pelvice. 2009; 1008-1015.
- Nurgül Örnek, MikailI nal, Özlem Banu Tulmaç, Zeynep Özcan-Dag and Kemal Örnek. Ocular blood flow in polycystic ovary syndrome.J Obstet Gynaecol Res. 2015.
- Peter W.Callen ,Md.5 Th Edition ,.Ultrasonography In Obstetrics And Gynecology. Isbn-13: 978-1416032649 ISBN-10: 1416032649 Edition: 5th.
- Parsanezhad ME, Bagheri MH, Alborzi S, Schmidt EH. Author information Ovarian stromal blood flow changes after laparoscopic ovarian cauterization in women with polycystic ovary syndrome. Hum Reprod.2003; 18(7): 1432-7.







