Anahita Kalirad1 and Seyedeh Nakisa Niknejad2*
1School of Medicine, Babol University of Medical Sciences, Babol, Iran.
2Department of Anatomical and Clinical Pathology, Cancer Institute, Tehran Univercity of Medical sciences, Tehran, Iran.
Corresponding Author E-mail: dr.nakisa_niknejad@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1068
Abstract
Colorectal cancer is the third and fourth major cause of cancer prevalence and also mortality among males and females, respectively in Iran. .The number of Lymph Nodes which are remain associated after surgery, are one of the pre-notice aspects which influence on patients. This study was a retrospective investigation and the studied population was the patients who had been hospitalized with colorectalcancer in Imam Khomeini Hospital, Tehran, Iran. Data on patient and tumor variables were collected from charts of 1095patients aged, diagnosed at our hospital in 2005-2014. 877 colorectal cancer patients were evaluated for involvement of lymph nodes during study. 495 subjects (56.4%) were male with the mean age of 51.34±12.23 and 382 patients (43.6%) were female with average age of 50.51±13.41. The mean number of evaluated lymph nodes in total period of the study was 8. The minimum number of involved lymph nodes in the patients was 0 and 91cases showed such situation and maximum number of involved lymph nodes in patients was 25 that included 1.03% of the patients. with regards to importance of definition of involved lymph nodes in prognosis and chance of survival in the patients and also suggestion of national cancer institute to define at least 12 lymph nodes for determination of disease stage, it is advised that definition of involved lymph nodes conduct with higher accuracy.
Keywords
Metastasized Lymph Nodes; Surveyed Lymph Nodes; Colorectal Cancer
Download this article as:| Copy the following to cite this article: Kalirad A, Niknejad S. N. Lymph Node Evaluation in Colorectal Cancer Patients in Iran. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Kalirad A, Niknejad S. N. Lymph Node Evaluation in Colorectal Cancer Patients in Iran. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=10534 |
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second in women worldwide1. It should be mentioned that a suitable evaluation of the lymph-node status of colorectal carcinoma specimens is important to comply with the suggestions of the UICC and also, to decide about the application of adjuvant therapy in patients with positive lymph nodes. In the last few years, immunohistochemical and molecular methods have been utilized by pathologists and surgeons for the identification of micrometastases, as the tumor, spread to the locoregional lymph nodes, has been widely regarded as the principle prognostic element2-5.
A sufficient retrieval and assessment of colorectal mesenteric lymph nodes is crucial to ensure which lymph nodes do not contain metastatic disease. It is clear that failure to examine enough lymph nodes might result in a failure in identifying the patients in whom a comparatively small fraction of the nodes is involved with cancer. Moreover, lymph node metastasis might happen in patients, irrespective of the T stage or the other pathologic factors6, 7.
In non-metastatic colorectal cancer, lymph node status is the strongest pathologic predictor of patient outcome. Around 68% of the patients, with no lymph node involvement, will survive 5 years, compared to only 40% of those with lymph node metastasis8.
The suggestions, offered by AJCC, ASCO, ACoS-CoC, CAP, and NCCN, expressed that the evaluation of ≥ 12 LNs is adequate forstaging a patient with CRC and these recommendations appeared to be able to put an end to the existing debate. However, the anecdotal evidence has indicated that such advices might not be implemented9.In present study, we focused on the frequency of CRC and involved lymph nodes and adequacy of lymph node dissection in patients who referred to our hospital in Tehran, Iran.
Patients and methods
Study design
This descriptive cross sectional study was performed on profiles of 1095 colorectal patients referred to radiotherapy and oncology unit of Imam Khomeini Hospital, Tehran, Iran during May 2005 to April 2014 were evaluated. From these profiles, those that lacked after surgery pathology report were excreted from study and 877 remained profiles were evaluated. The age and gender of patient and the number of evaluated lymph nodes abd the number of involved lymph nodes were recorded in this study.
The frequency of total number of colorectal patients was evaluated for each year and the frequency of numbers of evaluated lymph nodes in pathology report was evaluated for each year and the mean number of total evaluated lymph nodes in pathology report was evaluated in all years in our study.
To estimate the trends in the incidence rates, these registries’ data have pooled together. The ages have categorized into eight age groups (≤ 20 ; 21-30; 31-40; 41-50; 51-60; 61-70; 70<).
Statistical Analysis
Data have analyzed using SPSS, version 18. Chi-square test has employed to compare the scores of each of the measures and some of the parameters. A p-value of less than 0.05 has considered statistically significant.
Results
877 colorectal cancer patients were evaluated for involvement of lymph nodes during 2005-2014. 495 subjects (56.4%) were men with the mean age of 51.34±12.23 and 382 patients (43.6%) were women with average age of 50.51±13.41. No significant correlation was observed between involvement rate of lymph nodes and gender (p>0.05).
Table 1: the distribution of patients according to age
| Percent | Number | Age (year) |
| 1.49 | 13 | <20 |
| 6.16 | 54 | 21-30 |
| 15.39 | 135 | 31-40 |
| 21.89 | 192 | 41-50 |
| 22.58 | 198 | 51-60 |
| 17.44 | 153 | 61-70 |
| 15.05 | 132 | >70 |
| 100 | 585 | Total |
The mean number of evaluated lymph nodes in total period of the study was 8. The minimum number of involved lymph nodes in the patients was 0 (10%) and 91 cases showed such situation and maximum number of involved lymph nodes in patients was 25 that included 1.03% of the patients. Diagram 1 shows the evaluated lymph nodes separately.
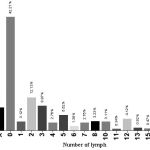 |
Figure 1: the distribution of evaluated lymph nodes based on frequency |
The mean number of involved lymph nodes in total period of the study was 7. The minimum number of involved lymph nodes in total period of study was zero that included 42.21% of cases (370 cases) and the maximum number of involved lymph nodes was 20 lymph nodes that included 0.32% (3cases) of the patients. Diagram 2 shows the rate of involved lymph nodes separately.
The minimum frequency of the patients was related to year 2007 (46 cases) and the maximum frequency was related to year 2014 (168 cases). The mean number of evaluated and involved lymph nodes is presented in table 2 for each year.
Table 2: shows the number of patients based on gender, the evaluated and involved lymph nodes for each year.
| Total
Number (%) |
Female
Number (%) |
Male
Number (%) |
Mean of
Involved lymph nodes /Evaluated lymph nodes |
Year of diagnosis
|
| 29 (4.96) | 11 (37.93) | 18 (62.07) | 3 / 10 | 2005 |
| 29 (4.96) | 15 (51.72) | 14 (48.28) | 3 / 8 | 2006 |
| 21 (3.59) | 5 (23.81) | 16 (76.19) | 4 / 7 | 2007 |
| 40 (6.84) | 15 (37.5) | 25 (62.5) | 2 / 7 | 2008 |
| 41 (7.01) | 20 (48.78) | 21 (51.22) | 3 / 8 | 2009 |
| 66 (11.28) | 33 (50) | 33 (50) | 2 / 7 | 2010 |
| 65 (11.11) | 27 (41.54) | 38 (58.46) | 3 / 8 | 2011 |
| 90 (15.38) | 34 (37.78) | 56 (62.22) | 2 / 7 | 2012 |
| 99 (16.92) | 47 (47.47) | 52 (52.53) | 3 / 10 | 2013 |
| 105 (17.95) | 48 (45.61) | 57 (54.39) | 3 / 8 | 2014 |
Discussion
An accurate examination of lymph node status in patients with non-metastatic colorectal cancer is evidently important. Lymph node status is the best predictor of long-term outcome in patients with colorectal cancer, who do not have metastatic disease. In fact, the existence of positive lymph nodes is used to determine the need for adjuvant chemotherapy of patients with colon cancer and is associated with an improved use of adjuvant radiation and chemotherapy for patients with rectal cancer10, 11. It should be mentioned that an inadequate lymph node evaluation is accompanied with a worse outcome, in terms of tumor recurrence and patient survival12-15. The basis of this relationship has not been recognized yet; however, it probably reflects an incorrect staging and the ensuing insufficient adjuvant therapy. So far, several authors have recommended that the patients, who are considered lymph node-negative on the basis of a low number of retrieved lymph nodes, should be considered at high risk of recurrence and thus, as candidates for adjuvant therapy. In fact, the retrieval of a low number of lymph nodes is usually likely to be an indicator of a poor-quality surgical or pathologic care14, 15
In the present study, 585 colorectal cancer patients were evaluated,495 subjects (56.4%) were male and 382 patients (43.6%) were female. In this investigation, the men-to-women ratio was 1.29 to 1 and the results were consistent with the previous studies16-18. In this study, the majority (77%) of colorectal cancer incidences were observed in the ages higher than 40 years. This finding is in parallel with some previous studies that indicated the incidence of colorectal cancer, higher in patients older than 45 years19-21. It is worth stating that the number of affected people per year has increased during 2005-2014. The reason might be the increase in visiting the medical centers, or the increased knowledge of people about the symptoms of this disease, or the recent developments of the diagnostic facilities, or a higher incidence of this disease in comparison with the past years22, 23.
The number of involved lymph nodes is one of the most important and effective factors in determining the prognosis of this disease and finding a treatment program for the patients. According to the studies by Jass and based on the guidelines of the international gut and chest surgery association, it has been proposed to evaluate, at least, 12 lymph nodes in order to be able to report the lymph nodes as negative23, 24.
The mean number of evaluated lymph nodes, in this study, was 8 lymph nodes, which is lesser in comparison with the other studies such as Wong et al., who evaluated 11 lymph nodes on average, or Tepper et al., who assessed 14 lymph nodes on average14, 25. In addition, in the analysis of INT-0089, 18 lymph nodes were evaluated. The mean number in the present study was lesser than the aforementioned suggestion for evaluating, at least, 12 lymph nodes to determine the disease stage26, 27. This mighthave beenresultedfrom an insufficient extraction of the tissue by the surgeon or an insufficient evaluation of the sample by the pathologist that leads to an inability for correct staging of the disease and also, for determining an appropriate treatment program for these patients. It will ultimately lead to over-treatment of the patients by radiotherapy and chemotherapy, because of the inability for distinct determination of the negativity of involved lymph-nodes.
In this study, in 578 cases (65.9%), less than 12 lymph nodes were evaluated, among which 234 subjects (26.7% of the total subjects) had zero involved lymph nodes and 74 cases (8.43% of the total subjects) had unknown numbers of involved lymph nodes (i.e., X lymph nodes). This means that in total, 37.1% of the entire studied cases,or in other words, 56.2% of the cases with less than 12 evaluated lymph nodes, did not possess a complete staging. Consequently, since it was unknown whether they have involved lymph nodes or not, they experienced over-treatment with radiotherapy and chemotherapy. Such a situation would cause financial expenses and other side effects on patients.
Although the remained 271 cases, with the evaluated lymph nodes of less than 12, did not have a complete staging, because of the presence of involved lymph nodes and also, the likelihood of involvement of more lymph nodes, they went under additional treatments with a higher certainty.
In the remained 318 cases, among the subjects in which the number of involved lymph nodes was equal or higher than 12, it was possible to correctly categorize the disease and define the lymph nodes’ involvement positive or negative, and to appropriately make an accurate decision about the necessity of additional treatments, including chemotherapy or radiotherapy.
In an evaluation, conducted by international gut and chest surgery protection institute on 844 patients, the depth of tumor and the number of positive lymph nodes were indicated as the two effective factors on the prognosis of the patients. The results of their study showed that the patients with positive lymph nodes, at the ratio of 1 to 4, had a better prognosis compared to the patients with a higher ratio of involved lymph nodes22.
A widespread analysis was performed on the results of the lymph nodes’ samples among the patients, participated in an inter-group evaluation of INT-0089. The analyses were conducted separately on the positive lymph node group (2,768 patients) and the negative lymph node group (698 patients). The mean number of reported lymph nodes in searchable patients was 11 (the range was from 1 to 87). These results demonstrated that the survival rate, independent of the disease, showed a significant decrease with a higher number of involved lymph nodes. The survival rate increased after controlling the number of involved lymph nodes, and even in the patients with negative lymph nodes, the survival rate was normally increased and the chance of cancer was significantly also increased25.
Conclusion
With regard to the importance of defining involved lymph nodes in the prognosis,and based on the chance of survival in patients and according to the suggestion of the national cancer institute for defining, at least, 12 lymph nodes for the determination of the disease stage, it is advised to provide the definition of involved lymph nodes with a higher accuracy. It should be also declared that this study was subjected to a number of limitations, such as the lack of stage determination, life-span determination, and survival of patients after treatment. Thus, it is strongly suggested to conduct further studies on these limitations and appraisethem.
Financial Disclosure
There is no conflict of interest
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893-2917.
- Liefers G-J, Cleton-Jansen A-M, van de Velde CJ, et al. Micrometastases and survival in stage II colorectal cancer. New England Journal of Medicine. 1998;339(4):223-228.
- Arnold MW, Young DM, Hitchcock CL, Barberá-Guillem E, Nieroda C, Martin Jr EW. Staging of colorectal cancer: Biologyvs. morphology. Diseases of the colon & rectum. 1998;41(12):1482-1487.
- Jeffers MD, O’Dowd GM, Mulcahy H, Stagg M, O’Donoghue DP, Toner M. The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. The Journal of pathology. 1994;172(2):183-187.
- Wong JH, Bowles BJ, Bueno R, Shimizu D. Impact of the number of negative nodes on disease-free survival in colorectal cancer patients. Diseases of the colon & rectum. 2002;45(10):1341-1348.
- Davidson B, Boulos P, Sams V, Styles J, Deane C. Detection of occult nodal metastases in patients with colorectal carcinoma. Cancer. 1990;65(4):967-970.
- Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Diseases of the colon& 2002;45(2):200-206.
- Soreide O, Norstein J. Rectal cancer surgery: optimisation—standardisation—documentation: Springer Science & Business Media; 2012.
- Shanmugam C, Hines RB, Jhala NC, et al. Evaluation of lymph node numbers for adequate staging of Stage II and III colon cancer. J Hematol Oncol. 2011;4(1):25.
- Chau I, Cunningham D. Adjuvant therapy in colon cancer: current status and future directions. Cancer treatment reviews. 2002;28(5):223-236.
- Schrag D, Gelfand SE, Bach PB, GuillemJ, Minsky BD, Begg CB. Who gets adjuvant treatment for stage II and III rectal cancer? Insight from surveillance, epidemiology, and end results–Medicare. Journal of clinical oncology. 2001;19(17):3712-3718.
- Le Voyer T, Sigurdson E, Hanlon A, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. Journal of clinical oncology. 2003;21(15):2912-2919.
- Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Annals of surgical oncology. 2003;10(1):65-71.
- Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. Journal ofClinical Oncology. 2001;19(1):157-163.
- Prandi M, Lionetto R, Bini A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Annals of surgery. 2002;235(4):458-463.
- Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. Jama. 2006;295(6):643-654.
- Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. Journal of the National Cancer Institute. 2005;97(3):219-225.
- Park YJ, Shin K-H, Park J-G. Risk of gastric cancer in hereditary nonpolyposis colorectal cancer in Korea. Clinical Cancer Research. 2000;6(8):2994-2998.
- Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta‐analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Alimentary pharmacology & therapeutics. 2009;30(2):113-125.
- Spencer EA, Key TJ, Appleby PN, et al. Meat, poultry and fish and risk of colorectal cancer: pooled analysis of data from the UK dietary cohort consortium. Cancer causes & control. 2010;21(9):1417-1
- Cross SS, Feeley KM, Angel CA. The effect of four interventions on the informational content of histopathology reports of resected colorectal carcinomas. Journal of clinical pathology. 1998;51(6):481-482.
- Wolmark N, Fisher B, Wieand HS. The prognostic value of the modifications of the Dukes’ C class of colorectal cancer. An analysis of the NSABP clinical trials. Annals of surgery. 1986;203(2):115.
- Jass J. Lymphocytic infiltration and survival in rectal cancer. Journal of clinical pathology. 1986;39(6):585-589.
- Jass J. Jass’ classification revisited. Journal of the American College of Surgeons. 1995;180(2):252-253.
- Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma. Journal of clinical oncology. 1999;17(9):2896-2896.
- Joseph NE, Sigurdson ER, Hanlon AL, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Annals of surgical oncology. 2003;10(3):213-218.
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. Journal of the National Cancer Institute. 2001;93(8):583-596.








