Oka Adi Parwata*1 , Sukardiman2, Mulya Hadi Santosa2 and Ida Ayu Alit Widhiartini3
1Nature Materials Study Group, Laboratory of Organic Chemistry, Department of Chemistry, Faculty of Mathematics and Natural Sciences Udayana University, Bali, Indonesia.
2Laboratory of Phytochemistry and Pharmacognosy, Faculty of Pharmacy Airlangga University, Surabaya, Indonesia.
3Departement of Pharmacy, Faculty of Medicine, Udayana University, Bali, Indonesia.
Corresponding Author E-mail : m.andita@yahoo.co.id
DOI : https://dx.doi.org/10.13005/bpj/1033
Abstract
This paper discusses the anticancer activity of 5-hydroxy-7-ethoxy-flavanones isolated from Kaempferia pandurata Roxb against mice fibrosarcoma resulted from benzopyrene induction. This study was initiated by isolating the 5-hydroxy-7-ethoxy-flavanones in the rhizome powder of Kaempferia pandurata Roxb followed by creating the fibrosarcoma in the nape of the mice through benzopyrene injection. Fibrosarcoma appeared approximately 1.5 months after the injection of benzopyrene. The mice with fibrosarcoma were then grouped into 3 treatment groups, namely: group I (negative control) those that were fed aquadest and CMC-Na only, group II those that were given 5-hydroxy-7-ethoxy-flavanones solution at a dose 80 of mg/kg body weight and group III those that were given the anticancer drug of at a dose of 13.33 mg/kg body weight. The treatment was carried out for about 1.5 months until the fibrosarcoma volume was about 100 mm3. The analysis was started with the surgery of the fibroarcoma tissue in the infected mice and then weighing out the fibrosarcoma obtained. The identification results of the isolates with UV-Vis, IR, Hydrogen and Carbon NMR, MS and three-dimensional X-ray diffraction suggested that the isolates was the compound of 5-hydroxy-7-ethoxy-flavanones or 5-hidroxy-7-methoxy-flavanon. The fibrosarcoma tissue was then stored in a container containing 10% formalin. The result of histopathological analysis with Hematoxilin and Eosin (HE) on the fibrosarcoma tissue showed the existence of polichromatin. This suggested that there was a damage in the cells caused by fibrosarcoma infection. The weighing results of the cancer cells showed that the oral concentration of 5-hydroxy-7-ethoxy-flavanones of 80 mg/kg could inhibit the growth of fibrosarcoma with the mass of 68.62 % and with the cancer drug (control +) there was a resistance of 95.95 % compared to the negative control which was only given CMC-Na orally. The results of this study indicated that 5-hydroxy-7-ethoxy-flavanones is potential to developed as a cancer chemotherapy.
Keywords
5-hydroxy-7-ethoxy-flavanones ; Kaempferia pandurata Roxb; benzopyren; fibrosarcoma
Download this article as:| Copy the following to cite this article: Parwata O. A, Sukardiman S, Santosa M. H, Widhiartini I. A. A. Inhibition of Fibrosarcoma Growth by 5-hydroxy-7-ethoxy-flavanons from Kaempferia pandurata Roxb. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Parwata O. A, Sukardiman S, Santosa M. H, Widhiartini I. A. A. Inhibition of Fibrosarcoma Growth by 5-hydroxy-7-ethoxy-flavanons from Kaempferia pandurata Roxb. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=10729 |
Introduction
As a traditional medicine, the rhizome of Kaempferia pandurata Roxb is used as a medicine for dry cough, ulcers, disorders of the colon, stomach swelling, inflammation of the uterus, diare, and cancer (Hembing , 1994).
The rhizome of Kaempferia pandurata Roxb extracted with diethyl ether produces relatively a large amount of 5-hydroxy-7-ethoxy-flavanones i.e. about 2,5% and alpinetin which is approximately 1%. 5-hydroxy-7-ethoxy-flavanones shows an antioxidants activity and relaxes smooth muscle. The high 5-hydroxy-7-ethoxy-flavanones content in the Kaempferia pandurata Roxb rhizome causes the isolation of the compound as a pure substance can be carried out easily and quickly. The 5-hydroxy-7-ethoxy-flavanones structure that the polarity reduces as a result of the existence of intramolecular hydrogen bonding between the carbonyl group at C-4 with a hydroxyl group at C-5, could be extractied with the application of semipolar or nonpolar solvents such as ethylacetate and CCl4 (Parwata,1998). 5-hydroxy-7-ethoxy-flavanones shows a fairly high toxicity with LC50 = 63.5 ppm (Parwata,2000). This compounds have an activity in inhibiting the growth of human breast cancer cells through the barrier activity of DNA topoisomerase I enzyme (Sukardiman et.al.,2006).
The death of the DNA of a cancer cells can affect the the process inside the cell, especially the process of cell replication and terminated with the death of cancer cells by apoptosis (Sukardiman, et.al.,2006). DNA damage cells is thought to activate tumor suppressor gene p53 besides inducing apoptosis (Whibley, 2009).
Activation and stabilization of p53 products by a flavonoid, is thought to inhibit the angiogenesis effect through the mechanism of down regulation by the decrease of VEGF expression, COX-2 and MMP-9 that involve in the metastasis process of cancer cells.. Based on this phenomenon the researchers suspect that 5-hydroxy-7-ethoxy-flavanones compound has the same activity as a therapy or chemopreventive to cancer on fibrosarkoma.
Materials and Methods
The materials used were as follows: rhizome Kaempferia pandurata Roxb powder obtained from Badung Market, Denpasar-Bali and male mice of 2 months old and weight of 20-25 grams. The chemicals used were: technical n-heksana, n-heksana (E Merck), methanol (E Merck), ethanol (E Merck), chloroform (E Merck), ethyl acetat (E Merck) aquadest, silica gel and Plate of Thin Layer Chromatography (TLC) Aluminium with silica Gel GF-254, E Merck), primary and secondary antibodies p53 and VEGF, buffered formalin 10 %, PBS, DAB, H2O2 3% and destilate water (DW).
The apparatus used were HPLC (High Peformance Liquid Cromatography) and TLC (Thin Layer Cromatography)-Densitometer Shimadzu CS-930, UV-Vis Spectrophotometer Hitachi 557 and 365 NIR Shimadzu, IR Spectrophotometer Jasco 5300 FTIR, proton NMR spectrometers and Carbon NMR Hitachi FT-1500 and Mass Spectrometer HP 8890, light microscope, polilysin preparate and objects glass
Isolation and identification of 5-hydroxy-7-ethoxy-flavanones from the rhizome of Kaempferia pandurata Roxb
Approximately 2000 grams of the powder of Kaempferia pandurata Roxb rhizome, was macerated with n-hexane for 24 hours, the liquid maserat obtained was then evaporated by rotary evavorator. The n- hexane extract was allowed to arise crystalline amorphous. After that, the amorphous crystals was chromatographed with vacuum column with n- hexane as the developer. The results of this column chromatography were collected and then reevaporated with rotary evavorator until the crystals appeared. The crystals were filtered, then recrystallized with methanol 3 times. The crystals obtained in this study were shiny white. In the next step, the melting point of the crystals was determined by DTA and Melting Points Apparatus Fiesher & John and then its purity was tested by TLC (Thin Layer Chromatography) with the application of some eluents of various polarities, TLC-densitometer with multiple wavelengths as well as HPLC (High Performance Liquid Chromatography). When the test shows that 5-hydroxy-7-ethoxy-flavanones was pure, the work was continued with subsequent identification by UV- Vis spectroscopy with sliding reagents, IR, 13C and 1H NMR, MS and three-dimensional X-ray diffraction to find out the structure of 5-hydroxy-7-ethoxy-flavanones.
Establishment of Fibrosarkoma on Mice by Benzopyrene Induction
Forty male mice were prepared to undergo a process of the research adaptation, then the mice had benzopyrene induction as much as 0.3 mg/0.2 mL in the oleum olivarium as a subcutaneous injection in the scapular area 5 times every two days. All mice were maintained in the same atmosphere and diet for two months/until the cancer formed in the neck. After the cancer volume reached ± 100 mm3, the mice with cancer were randomly divided into 3 groups. Group I was the negative control which was only given CMC-Na. The second group was given 5-hydroxy-7-ethoxy-flavanones at a dose of 80 mg/kg; group III was given cyclophosphamide at a dose of 13.33 mg/kg. All of the test materials were administered orally and were given every day for 14 days. After that the mice were surgered for removing the fibrosarcoma tissue followed by weighing the tissue obtained.
Results And Discussion
Result
Isolation of 5-hydroxy-7-ethoxy-flavanones
Isolates obtained was in the form of needles white crystalline. Analysis of purity was performed by TLC using the mobile phase that different polarity. The results obtained showed one stain as shown in Table 1 below :
Table 1: Analysis of purity isolates by Thin Layer Chromatography
| No. | Mobile phase | Result |
| 1 | BAW = 4:1:5 | 1 stain |
| 2 | CHCl3:CH3CH2OH=3:1 | 1 stain |
| 3 | CHCl3:CH3CH2OH=15:1 | 1 stain |
| 4 | nCH3CH2CH2CH2CH2CH3:CH3COOCH2CH3 =1:1 | 1 stain |
| 5 | nCH3CH2CH2CH2CH2CH3:CH3COOCH2CH3 =3:1 | 1 stain |
| 6 | nCH3CH2CH2CH2CH2CH3 : CH3COOCH2CH3= 3 : 2 | 1 stain |
| 7 | CH3CH2CH2CH2CH2CH3 : CH3COOCH2CH3 = 7: 3 | 1 stain |
Note : BAW : n-Butanol : Acetic acid : Water All the Mobile phase result one stain Analysis of purity by TLC-densitometry at a wavelength [220 nm (I); 254 nm (II); 287 nm (III); 300 nm (IV); 320 nm (V) and 350 nm (VI)] resulted in one peak whereas by HPLC the purity of the compound obtained was of 94.83%. All this purity test results showed that the isolate was pure chromatographic Analysis of the melting point showed that the range of its melting point = 99-100oC (I); 99,5-101.5oC (II) and 99.5-100.5o C (III), whereas with DTA melting point observed was of 97oC, and the substance stability was up to a temperature of 150oC. These results indicated that the isolates were pure based on its physical properties. Analysis of chemical constituents showed that isolates indicated positive flavonoids of flavanones, as shown by the following Table 2 :
Table 2: Kualitatif Chemical Ingredients of isolates
| No. | Reagent | Discoloration | Indicated |
| 1. | Willstater | Colorless to pink colour | (+) Flavonoid |
| 2. | NaOH 10% | Colorless to pink colour | (+) Flavonoid |
| 3. | FeCl3 | Colorless to pink colour | (+) Flavonoid |
Note : All the Reagent result flavonoid
Identification with UV-Vis spectrophotometer showed the l max = 287 nm (band II) and shoulder = 325 nm (band I).
Subsequent analysis when isolates in methanol was coupled with AlCl3, the absorption band II has a bathochromic shift of 23 nm. Identification by IR spectroscopy showed some peaks as shown by the data in the following Table 3 :
Table 3: Identification isolates by IR spectroscopy
| No. | Wavenumber (cm-1) | Indicated |
| 1 | 3437.46 | -OH at C-5 |
| 2 | 3061.31 | -C-H stretch of aromatics |
| 3 | 1645.43 | -C=O ketones at C-4 |
| 4 | 1622.26 and 1579.64 | -C=C- of aromatic |
| 5 | 3000-2800 | -C-H of methyl |
| 6 | 1450-1350 | -C-H of methylene |
| 7 | 1250 and 1290 | -C-O |
Identification Isolates by Proton NMR spectroscopy resulted some peaks as shown by the data in the following Table 4 :
Table 4: Identification Isolates by Proton NMR spectroscopy
| No. | Chemical shift (d), ppm | Indicated |
| 1 | 3.75 (s) | ArOCH3 (8H) |
| 2 | 6.019 (s) | H-6 and H-8 (2H) |
| 3 | 11.948 (s) | -OH (1H) |
| 4 | 2.817 to 3.029 (m) | H-3 cis or trans (2H) |
| 5 | 5,284 | H-2 (1H) |
Identification Isolates by Carbon NMR spectroscopy resulted some peaks as shown by the data in the following Table 5 :
Table 5: Identification Isolates by Karbon NMR spectroscopy
| No. | Chemical shift (d), ppm | Indicated |
| 1 | 55.583 | O-CH3 (1C) |
| 2 | 79.121 | C-2 (1C) |
| 3 | 43.326 | C-3 (1C) |
| 4 | 195.440 | C-4 (1C) |
| 5 | 163.944 | C-5 (1C) |
| 6 | 95.037 | C-6 (1C) |
| 7 | 167.755 | C-7 (1C) |
| 8 | 94.153 | C-8 (1C) |
| 9 | 162, 572 | C-9 (1C) |
| 10 | 103.025 | C-10 (1C) |
| 11 | 138.241 | C1 ‘(1C) |
| 12 | 138.241 | C2’and C6 ‘(2C) |
| 13 | 128.667 | C3′-C5 ‘(3C) |
Analysis of isolates by mass spectroscopy resulted in molecular ion with m/e=270 and the fragmentation as shown by the data in the following Table 6 :
Table 6: Identification Isolates by Massa spectroscopy
| No. | m/e | Indicated |
| 1 | 270 | M+ |
| 2 | 252 | M +– H2O |
| 3 | 242 | M+`-CO |
| 4 | 227 | M +-CO -CH3 |
| 5 | 213 | M +-CO- C2H5 |
| 6 | 193 | release of ring B/phenyl |
| 7 | 166 | M+-C2H5-C6H5 |
| 8 | 152 | M+ -C2H5-C6H5-CH3 |
| 9 | 123 | M+-C2H5-C6H5-CO-CH3 |
| 10 | 77 | C6H5 |
Note : M+ indicated Massa Molecole Relatif of 5-hydroxy-7-ethoxy-flavanones
Surgery of fibrosarcoma
After one month of benzopyrene injection, fibrosarcoma started to grow and approximately after 2 months the volume of fibrosarcoma was 100 mm3. Prior to the treatment, one of the infected mice was sacrificed to prove the existence of fibrosarcoma. Justification of this was carried out by observing the histopathological tissue of the fibrosarcoma with HE colouring. The weight of the surgical result obtained was 3.4756 grams and the histopatology observation with 400x magnification showed a lot of chromatin (polychromatin) proving the damage of the cells due to fibrosarcoma as shown by the following After one month of benzopyrene injection, fibrosarcoma started to grow and approximately after 2 months the volume of fibrosarcoma was 100 mm3. Prior to the treatment, one of the infected mice was sacrificed to prove the existence of fibrosarcoma. Justification of this was carried out by observing the histopathological tissue of the fibrosarcoma with HE colouring. The weight of the surgical result obtained was 3.4756 grams and the histopatology observation with 400x magnification showed a lot of chromatin (polychromatin), as shown by the following figure 6
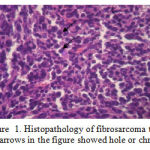 |
Figure 1: Histopathology of fibrosarcoma tissue |
(Note : arrows in the figure showed hole or chromatin)
After 14 days of treatment, the mice with fibrosarcoma were sacrificed to take the cancer tissue for subsequent examination i.e. the weight of the cancer tissue, as shown by the data in the following table :
Table 7: The Weight of Fibrosarkoma Mice after Treatment
| Type of treatment | Fibrosarkoma Weight (grams) | ||||
| I | II | III | IV | Average | |
| without treatment | 3.4756 | 3.4557 | 3.4656±0.01 | ||
| CMC-Na [ Control (-)] | 4.2765 | 4.1665 | 4.1095 | 4.1383 | 4.1727±0.07 |
| 5-hydroxy-7-ethoxy-flavanones 80 mm/kg BW | 1.0665 | 1.0605 | 1.1198 | 1.1027 | 1.0874±0.02 |
| Cyclophosphamide [ Control ( + ) ] | 0.1709 | 0.1021 | 0.2553 | 0.1021 | 0.1576±0.07 |
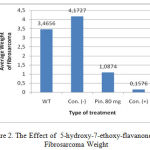 |
Figure 2: The Effect of 5-hydroxy-7-ethoxy-flavanones on Fibrosarcoma Weight |
Discussion
Isolates obtained was in the form of needles white crystalline. Purity analysis with chromatographic by TLC , TLC – densitometry and HPLC showed one peak with a stain and a different polarity eluent. This shows that all this purity test results showed that the isolate was pure chromatographic. Analysis of one of the physical properties of the isolates are melting with Melting Points Apparatus and DTA showed that isolates said to be purely due to their melting point range < 2o. Based on these results further isolates identified spectroscopically. Phytochemical screening reagents Wilstatter color, FeCl3 and NaOH 10% indicated that the isolates do indeed flavonoids flavanones. Identification with UV-Vis spectrophotometer showed the l max = 287 nm (band II) and shoulder = 325 nm (band I). This was in agreement to the literature in which flavonoids flavanones show two absorption bands i.e. between 275-295 nm (band II) and a shoulder at 300-330 nm that caused by the cinnamoyl and benzoyl groups of flavanones as shown in the structure below (Silverstein et al , 1981; Parwata, 1998):
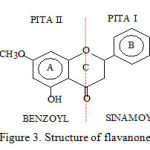 |
Figure 3: Structure of flavanone |
Bathochromic shift indicates that flavonoids flavanones contains an -OH group at C-5 as shown in the structure below (Silverstein et.al.,1981):
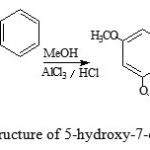 |
Figure 4: Structure of 5-hydroxy-7-ethoxy-flavanones |
Identification by IR spectroscopy proved that isolates containing -OH groups, -CH, -C=O and -C-O
The results of the Proton and Carbon NMR spectra interpretation showed that 5-hydroxy-7-ethoxy-flavanones has 14 H atoms and 16 C, as shown by the following structure :
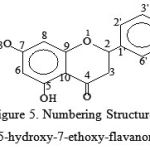 |
Figure 5: Numbering Structure of 5-hydroxy-7-ethoxy-flavanones |
Analysis with mass spectroscopy resulted in molecular ion with m/e =270 that indicated the relative molecular mass of 5-hydroxy-7-ethoxy-flavanones with some fragmentation. This is illustrated by the following fragmentation schemes (Silverstein et al , 1981)
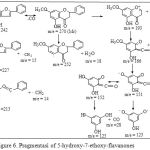 |
Figure 6: Pragmentasi of 5-hydroxy-7-ethoxy-flavanones |
The result of the three-dimensional X-ray diffraction analysis showed that isolates as 5-hydroxy-7-ethoxy-flavanones have a structure as follows:
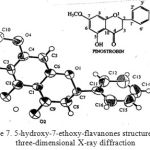 |
Figure 7: 5-hydroxy-7-ethoxy-flavanones structure with three-dimensional X-ray diffraction |
The weight of the surgical result obtained was 3.4756 grams and the histopatology observation with 400x magnification showed a lot of chromatin (polychromatin). proving the damage of the cells due to fibrosarcoma
After 14 days of treatment, the mice with fibrosarcoma were sacrificed to take the cancer tissue for subsequent examination i.e. the weight of the cancer tissue. The result obtained was that oral 5-hydroxy-7-ethoxy-flavanones concentration as much as of 80 mg/kg body weight could inhibit the growth of fibrosarcoma with a weight of 68.62% grams while with the cancer drug (control +) there was a barrier of 95.95% compared to the negative control which was only given CMC-Na orally. This suggested that 5-hydroxy-7-ethoxy-flavanones is potentially be developed as a cancer chemotherapeutic agent.
Conclusion
Based on the results of the research, it can be concluded as follows :
Isolates produced are correct is 5-hydroxy-7-ethoxy-flavanones
Results of histopathological analysis fibrosarcoma cells with HE staining showed there are many chromatin on fibrosarcoma as evidence of the destruction of normal cells due to the occurrence of fibrosarcoma
5-hydroxy-7-ethoxy-flavanones has a significant effect in inhibiting the growth of fibrosarcoma.
5-hydroxy-7-ethoxy-flavanones oral concentration of 80 mg/kg body weight could reduce as much as 68.62 % fibrosarcoma weight while with a cancer drug (control +) there was adecrease of 95.95 % , this suggested that 5-hydroxy-7-ethoxy-flavanones is potentially be developed as a cancer chemotherapy agent
Suggestion
This research should be continued on the effect of the treatment of p53, COX-1, COX-2, VEGF and MMP-9
Acknowledgements
The author would like to thank profusely all parties, especially Mr. Eko Adiputra for his help in the maintenance and surgery of the mice in the Animals Laboratory, Faculty of. Pharmacy-Airlangga University during the research. We also thank profusely the Chief and Staff of LPPM Udayana University for providing the funds to afford this research.
References
- Adi Parwata, 1998, Isolasi, Identifikasi Senyawa 5-hydroxy-7-ethoxy-flavanones pada Rimpang Temu Kunci (Kaempferia pandurata Roxb) dan Standarisasi Ekstrak Etanol berdasarkan Kadar 5-hydroxy-7-ethoxy-flavanonesnya dengan KLT Densitometri. Thesis, PPS Unair, Surabaya.
- Adi Parwata, 2000,Uji Toksisitas Senyawa 5-hydroxy-7-ethoxy-flavanones pada Rimpag Temu Kunci (Kaempferia pandurata Roxb), DIK/DIKS, Lemlit Unud, Bali.
- Anonim, 2005, Fibrosarkoma, http://medika-online.blogspot.com/2005/11/ Fibrosarkoma.html , diakses, 28 September 2011
- Bail,L., Aubourg L., Habrioux G., 2000. Effect of 5-hydroxy-7-ethoxy-flavanones on estrogen metabolism and estrogen reseptor transactivivation, Cancer Lett, Aug, 1;156910;37-44.
- Banerjee S. , Carlos ,B.R and Bharat B.A.(2002) Suppression of 7,12-Dimethylbenz(a) anthracene-induced Mammary Carcinogenesis in Rats by Resveratrol , Carcinogenesis, 62, 4945-4954.
- Cumming,J., Smyth,J.F.,1993. DNA Topoisomerase I and II as target of Rational Design of New Anticancer Drugs, Ann Oncology, Aug, 3(7), 533-534.
- Harborne J.B. and Mabry, T.J., 1992, Flavonoids, Advances and Research, Chapman and Hall, London.
- Jianguo,M., Karin, Reed,A., and James,M., Gallo, 2002, Cells Designed to Deliver Anticancer Drug by Apoptosis, Cancer Research, 62, 1382-7.
- Manahan,D., and Wienberg,R.A., 2002. The Hallmarks of Cancer, Cell, 100 : 57-70. Markam K.R., 1988, Cara Identifikasi Flavonoid,terjemahanPenerbit ITB Bandung,1-59
- Nagata ,S. 1997. Apoptosis by Death Factor, Cell. 88 : 355 – 365.
- Pai,S., Kaustubh,D. and Debabrata,M., 2001. Central Role of p53 Regulation of Vascular Permeability Factor/Vascular Endothelial Growth Factor (VPF/VEGF) Expression in Mammary Carinoma, Cancer Research, 61, 6952-6957.
- Ravi,R.,Bijoyesh,M., Zaver M.B., Carrie. H.S., Dmitri,A., 2000. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α, Gene and Development, Vol 14.
- Silverstein, Bassler and Morrill, 1981, Spectrometric Identification of Organic Compounds, John Willey and Sons, New York.
- Sukardimanb, Darwanto A, Tanjung M., Parwarta Oka Adi ., 2000. Cytotoxic Mechanism of Flavonoid from Temu Kunci (Kaempferia pandurata) in Cells Culture of Human Mammary Carcinoma, Clinical Hemorheology and Microcirculation, 23 ,185-190.
- Sukardiman, Noor Cholies Z, Sismindari ,2006. Induksi Apoptosis dan Peningkatan Ekspresi p53, Bax serta Aktivasi Enzim Caspase Sel Kanker Payudara Manusia oleh 5-hydroxy-7-ethoxy-flavanones dari Kaempferia pandurata Roxb, Laporan Penelitian Hibah Bersaing Tahun I, Lembaga Penelitian Universitas Airlangga.
- Wittmann S, Bali P, Donapaty S, Nimmanapalli R, Guo F, Yamaguchi H, Huang M, Jove R, Wang HG & Bhalla K. (2003). Flavopiridol down-regulates antiapoptotic proteins and sensitizes human breast cancer cells to epothilone B-induced apoptosis. Cancer Res 63: 93−99.
- Whibley C., 2009, p53 polymorphisms: cancer implications, Nature RevIew Cancer, Vol. 9, Februari 2009, p. 95-107, http://www. google.co.id /p53 +cancer+search,Diakses, 6 Desember 2011.








