Manuscript accepted on :November 20, 2016
Published online on: --
Plagiarism Check: Yes
Huali Yang, Na Liu and Shaochin Lee
School of Life Sciences, Shanxi University, Taiyuan, Shanxi, PR China.
Corresponding Author E-mail: lee_shao@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1030
Abstract
The study investigated the underlying molecular mechanisms of ethanol extract of Annona muricata. L induced liver cancer cell apoptosis. The anti-proliferative effect of ethanol extract of Annona muricata. L was evaluated by MTT assay. Flow cytometry was used to detect the cell cycle and apoptosis. TUNEL assay was used to confirm cell apoptosis. DCFH-DA fluorescent probe can detect the level of intracellular reactive oxygen species (ROS). Different concentrations of ethanol extract of Annona muricata. L leaves (0, 30, 60, 120, 240 µg/ml) were used to treat liver cancer HepG2 cells for 24h or 48h, the cell viability was inhibited in time- and dose-dependent manners. Ethanol extract of Annona muricata. L leaves increased the number of cells in the sub-G1 fraction in a dose-dependent manner. TUNEL assay confirmed that the extract caused apoptosis of the cancer cells. Further analysis revealed that the extract produced reactive oxygen species (ROS). Antioxidant N-acetylcysteine was able to inhibit the extract-triggered ROS production and apoptosis. In conclusion, our results suggest that the ethanol extract of Annona muricata. L leaves induces HepG2 cell apoptosis through ROS pathway.
Keywords
Annona muricata. L; liver cancer cell; apoptosis; ROS
Download this article as:| Copy the following to cite this article: Yang H, Liu N, Lee S. Ethanol Extract of Annona Muricata. L Induces Liver Cancer Cell Apoptosis Through ROS Pathway. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Yang H, Liu N, Lee S. Ethanol Extract of Annona Muricata. L Induces Liver Cancer Cell Apoptosis Through ROS Pathway. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=10594 |
Introduction
Liver cancer is one of the common cancers world-wide, the incidence has been rising over years, which casts great socio-economic burdens to societies1. Chemotherapy is commonly used to treating cancers, including liver cancer. Due to some reasons such as the chemo-drug resistance, the outcome of current chemotherapy for liver cancer is often not desirable2,3. Therefore, it is necessary to look for more therapies for the treatment of liver cancer, including alternative or complementary therapies.
Natural plant extracts have drawn attentions for their multiple biological functions4. Annona muricata. L, also known as graviola, soursop and guanabana, is an evergreen tree that is seen in tropical and sub-tropical areas5. The plant is belonged to the Annonaceae or custard apple family and has been described as “cancer killer”6. The fruit trees are widely grown in many tropical zones and used as traditional medicine to treat cancer and tumors7. Despite the evidence for apoptotic effect of extracts of the plant, the underlying molecular mechanisms remain largely unknown. Therefore, we attempted to investigate the cytotoxicity and apoptosis effects as well as the underlying mechanisms of ethanol extract of Annona muricata. L leaves using liver cancer HepG2 cells as an experimental model.
Materials and Methods
Reagents
Dulbecco’s modified Eagle medium, Penicillin-streptomycin were purchased from Gibco (Grand Island, NY). 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT), Dimethyl sulfoxide (DMSO), N-acetylcysteine (NAC), 4′, 6-Diamidino-2-phenylindole (DAPI), Triton X-100 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was a product of Hyclone (Logan, UT, USA). Cell cycle analysis and quantification of reactive oxygen species (ROS) were performed using kits from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). TdT-mediated dUTP nick end labeling (TUNEL) assay kit was purchased from Roche (Mannheim, Germany).
Preparation of ethanol extract of Annona muricata. L leaves
Annona muricata. L leaves were collected from Nanning city, Guangxi province of China. The taxonomic identity was verified by our botanist. Leaves were air dried and cut into pieces of approximately 1cm×1cm in size. The leaf pieces were weighted and placed into glass bottles, and 70% ethanol was added to cover the leaf pieces. The bottles were placed in dark at room temperature for 10 days, after which the supernatants were collected by filtering through Whatman 3 mm filter paper and dried using a vacuum drier. The resulted powders were dissolved in DMSO and stored at room temperature in the dark till use.
Cell culture
Human hepatocarcinoma cell line (HepG2) cells were purchased from the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in medium supplemented with 10% FBS, 100 µg/ml streptomycin and 100 IU/ml penicillin in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA) with 5% CO2 at 37 o C.
Study groups
Control group: HepG2 cells were incubated in the culture medium and received DMSO alone. Treatment samples: HepG2 cells were incubated with various concentrations of ethanol extract of Annona muricata. L leaves (30, 60, 120, 240 µg/ml). Antioxidant group: HepG2 cells were pretreated with 5 mM NAC (1 h) before adding the ethanol extract of Annona muricata. L leaves.
Cell viability assay
Cell viability was measured using MTT assay. Cells were seeded to a 96-well culture plate, and cultured for 24 or 48 h with different concentrations of the extract. Then, MTT (0.5 mg/ml) was added to each well and the plate was incubated at 37 o C for 4 h. At the end of incubation, medium was removed and 150 µl DMSO was added. After gentle shaking of plate on shaker for 10 min, optical density of each well was determined at 490 nm using a microplate reader (SpectraMax M5, Molecular Devices, CA, USA)
Cell cycle assay
Cell cycle was analyzed using flow cytometry analysis. In brief, HepG2 cells were plated and culture in 6-well plates (1×105 cells/well). After treatment, cells were collected and centrifuged at 1000 g for 5 min and washed twice with cold 1×PBS. Then, cells were fixed with 70% cold ethanol and stored at -20 o C for overnight. Cells were stained with 0.5 ml of propidium iodide (PI) staining buffer, which contains 200 mg/ml RNase A, 50 µg/ml PI, at 37 o C water for 30 min in the dark .The stained cells were analyzed for DNA content using BD FACSCanto II flow cytometer (FACS Calibur, Becton Dickinson, CA, USA) and the percentage of cells in each phase of the cell cycle was evaluated using the ModFit software.
Tunel assay
TUNEL assay was performed with an assay kit. In brief, cells were cultured on the cover slide. After treatment, cells were washed twice with 1×PBS, fixed in 4% (w/v) paraformaldehyde in 1×PBS for 1 h at room temperature, washed twice with 1×PBS again. Then, the fixed cells were incubated with blocking solution (3% H2O2 in methanol) for 10 min and rinsed with 1×PBS. The cells were then incubated in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. 50 µl of reaction mixture containing TdT enzyme was added to the cells and they were all incubated for 1 h at 37 o C. Finally, sample was mounted with mounting solution containing DAPI. Fluorescence was visualized under fluorescence microscope (Olympus IX73, Tokyo, Japan).
Quantification of ROS
The level of intracellular ROS level was quantified using a ROS assay Kit. DCFH-DA, a fluorescent probe, is oxidized by ROS in viable cells to DCF. At the end of treatment, cells were incubated with 3 mM DCFH-DA for 30 min at 37 o C in the dark. Then the cells were washed with 1×PBS and analyzed immediately with a flow cytometer with excitation and emission settings of 488 nm and 530 nm, respectively. Alternatively, cells were transferred to 96-well plates for determination of intensity (optical density) of fluorescence using our plate reader.
Statistical analysis
All experiments were performed in triplicate. Data were analyzed by one way ANOVA analysis using the statistical software package SPSS. A p<0.05 was considered to be statistical significant.
Results
The ethanol extract of Annona muricata. L leaves reduces the cancer cell viability
The viability of cell was measured using MTT assay. As shown in Fig. 1, ethanol extract of Annona muricata. L leaves markedly inhibited the viability of the cells in dose- and time-dependent manners. The 24 and 48 h LD50 values were approximately 180 and 80 µg/ml respectively.
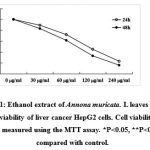 |
Figure 1: Ethanol extract of Annona muricata. L leaves inhibits the viability of liver cancer HepG2 cells. Cell viability was measured using the MTT assay. *P<0.05, **P<0.01 compared with control. |
Ethanol extract of Annona muricata. L leaves induces apoptosis
The cell cycle of HepG2 cells was evaluated by PI staining in combination with flow cytometry analysis. As shown in Fig. 2A, the herbal extract did not cause cell cycle arrest at G1, S or G2M phases. However, it increased the percentage of sub-G1 fraction in a dose-dependent manner (Fig. 2A and B); at the concentration of 120 µg/ml, it produced approximately 29% of sub-G1 cells. In TUNEL assay, many cells were found to be positive for the staining after they were treated by the herbal extract for 24 h. In contrast, much less cells in the control groups showed positivity (Fig. 2C).
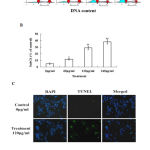 |
Figure 2:Ethanol extract of Annona muricata. L leaves causes apoptosis of liver cancer HepG2 cells. (A) The cell cycle was analysis by flow cytometry analysis. (B) The percentage of sub-G1 cells, which represents the amount of apoptotic cells. (C) The visualization of apoptotic cells using TUNEL staining: blue color indicates cell nuclei stained by DAPI and green color indicates TUNEL-positive cells which are apoptotic cells. *P<0.05, **P<0.01 compared with control.
|
The herbal extract increases the intracellular level of ROS
We analyzed whether the herbal extract could generate ROS in HepG2 cells by comparing ROS level amongst control, treatment (120 µg/ml of extract) and antioxidant (120 µg/ml extract plus 5 mM NAC) groups. As shown in Fig. 3A, after treatment for 2 h, the fluorescence intensity was increased when measured using fluorescent plate reader. Alternatively, the ROS level was analyzed with a flow cytometer (Fig. 3B). The result showed that the ROS level was significantly increased in the cancer cell treated with the extract. NAC was able to inhibit the extract-induced ROS (Fig. 3A and B).
 |
Figure 3: Ethanol extract of Annona muricata leaves increases the intracellular level of ROS and the inhibitory effect of NAC. ROS level was determined using (A) fluorescence intensity measurement and (B) flow cytometry analysis. DCFH-DA was used as the molecular probe. **P<0.01 compared with control.
|
NAC inhibits the extract-induced apoptosis
If the extract caused the cancer cell apoptosis through oxidative stress pathway, inhibition of ROS by NAC as shown in Fig. 3, should inhibit the extract-induced apoptosis. Indeed, as shown in Fig. 4, NAC was able to inhibit the extract-induced apoptosis of the cancer cells.
Discussion
The phenotypes of apoptosis include nuclear chromatin condensation, nuclear fragmentation, and formation of distinct apoptotic bodies8. A large number of evidence suggests that apoptosis induction in tumor cells is a major protective mechanism against the development and progression of cancer9. Annona muricata. L is an important source of different bioactive compounds such as antioxidants, antivirals, antimicrobials et al10,11. These antitumor activities can play a significant role in development of new pharmaceutical compounds as antitumor drugs. Our results revealed that the ethanol extract of Annona muricata. L leaves exhibited dose- and time-dependent anti-proliferation effects on HepG2 cells by promoting apoptosis, which suggests that the ethanol extract of Annona muricata. L leaves can be used as alternative or complementary treatment for liver cancer.
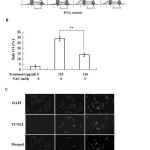 |
Figure 4: NAC inhibits the extract-induced apoptosis. (A) The cell cycle was analysis by flow cytometry technique. (B) The percentage of sub-G1 cells in antioxidant group is lower than treatment samples. (C) The TUNEL staining of apoptotic cells. **P<0.01 compared with control.
|
Increase in intracellular reactive oxygen species (ROS) production beyond the capacity of antioxidant defense system of cancer cells can lead to induction of apoptosis, which has been proposed as a strategy in anti-cancer therapy12,13. In the present study, the ethanol extract of Annona muricata. L leaves increased ROS generation, and induced the apoptosis. We use the antioxidant to inhibit the generation of ROS, which reversed the cell apoptosis. This indicates that the extract causes apoptosis through ROS pathway.
Since the 80s, scientists had done studies to identify components from Annona muricata; they had separated and identified more than 50 lactone compounds from the plant so far, including reticuline, coclaurine, N-p-coumaroyltyramine, Methyl caproic acid, calarene and so on. The plant has Annonaceous acetogenins which has a strong anti-cancer and antitumor activities, it can produce toxicity against tumor cells, such as lung, breast, and colon cancer cells14. Diterpenoid compounds were frequently isolated from the Annonaceae plants, most of which have strong anticancer activity. Cunabic acid and ent-kayran-19-al-17-oic acid were isolated from pound apple, when cunabic acid was over 5µmol/l and ent-kayran-19-al-17-oic acid was over 25µmol/l, it can inhibit the growth of liver cancer cell lines SMMC-7721 significantly in vitro15. Whether these compounds also exist in Annona muricata L. remains unknown. It will be interesting to isolate active components from the extract, and develop into main stream chemically defined drug(s) for classical chemotherapy.
In our previous study, we found that the ethanol extract of Annona muricata. L leaves induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway16. Therefore, we try to examine the relationship between ROS and endoplasmic reticulum (ER) stress. NAC did not inhibit the extract-induced ER stress. Similarly, inhibition of ER stress did not affect ROS production by the extract (figure not shown). These observations indicate that ROS and ER were not in a same signal transduction pathway, which suggests that the extract causes apoptosis by activating at least two pathways, the ROS and ER stress pathways simultaneously.
Conclusion
Our results have shown that the ethanol extract of Annona muricata. L leaves causes apoptosis of liver cancer HepG2 cells through ROS pathway.
Acknowlegements
The study was supported by direct grant from the Shanxi University and the department technology of Shanxi province (2015 and 2016).
References
- Wang, J., Yuan, L., Xiao, H., Xiao, C., Wang, Y., Liu, X. Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mito chondrial pathways. , 2013; 18(6): 751-65.
- Ma, S., Jiao, B., Liu, X., Yi, H., Kong, D., Gao, L., Zhao, G., Yang, Y., Liu, X. Approach to radiation therapy in hepatocellular carcinoma. Treat. Rev., 2010; 36(2): 157-163.
- Honda, K., Sbisa, E., Tullo, A., Papeo, P.A., Saccone, C., Poole, S., Pignatelli, M., Mitry, R.R., Ding, S., Isla, A. p53 mutation is a poor prognostic indicator for survival in patients with hepatocellular carcinoma undergoing surgical tumour ablation. J. Cancer., 1998; 77(5): 776-782.
- Efferth, T. Cancer therapy with natural products and medicinal plants. Med., 2010; 76(11): 1035-1036.
- Moghadamtousi, S.Z., Fadaeinasab, M., Nikzad, S., Mohan, G., Ali, H.M., Kadir, H.A. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. J. Mol. Sci., 2015; 16(7): 15625-15658.
- Baskar, R., Rajeswari, V., Kumar, T.S. In vitro antioxidant studies in leaves of Annona Indian. J. Exp. Biol., 2007; 45(5): 480-485.
- Moghadamtousi, S.Z., Kadir, H.A., Paydar, M., Rouhollahi, E., Karimian, H. Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB. Complement. Altern. Med., 2014; 14: 299.
- Maiuri, M.C., Zalckvar, E., Kimchi, A., Kroemer, G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Rev. Mol. Cell. Biol., 2007; 8(9): 741-752.
- Ghobrial, I.M., Witzig, T.E., Adjei, A.A. Targeting apoptosis pathways in cancer therapy. Cancer. J. Clin., 2005; 55(3): 178-194.
- Torres, M.P., Rachagani, S., Purohit, V., Pandey, P., Joshi, S., Moore, E.D., Johansson, S.L., Singh, P.K., Ganti, A.K., Batra, S.K. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Lett., 2012; 323(1): 29-40.
- Moghadamtousi, S.Z., Kadir, H.A., Paydar, M., Rouhollahi, E., Karimian, H. Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB. Complement. Altern. Med., 2014; 14: 299.
- Trachootham, D., Alexandre, J., Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Rev. Drug. Discov., 2009; 8(7): 579–591.
- Montero, A.J., Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs., 2011; 71(11): 1385–1396.
- Cheng, C.L. The research situation of Annona muricata chemical constituents and biological activities. Journal. of. tropical. Agriculture., 2001; 93(5): 62-70.
- Yang, Z. The research situation of the effect of Annona muricata against tumor cancer. Medical. and. Pharmaceutical. Journal., 2005; 9(7): 484-485.
- Liu, N., Yang, H.L., Wang, P., Lu, Y.C., Yang, Y.J., Wang, L., Lee, S.C. Functional proteomic analysis revels that the ethanol extract of Annona muricata L. induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J. Ethnopharmacol. 2016; 189: 210-217.







