Ramachandran Tamilselvi1, Rajendran Mathan Rajan2, Veronica Aruna Kumari3 and Deivanayagam Kandaswamy2
1Department of Conservative Dentistry and Endodontics, Sree Balaji Dental College and Hospital, Bharath University, Chennai, Tamilnadu, India.
2Department of Conservative Dentistry and Endodontics, Faculty of Dental Sciences Sri Ramachandra University, Chennai, Tamilnadu, India.
3Department of Conservative Dentistry and Endodontics, Madha Dental College and Hospital, Kundrathur, Chennai, Tamilnadu, India.
Corresponding Author E-mail : drtamil_chandran@yahoo.co.in /
DOI : https://dx.doi.org/10.13005/bpj/1055
Abstract
The study was performed to find the influence of organic matrix on hardness and fracture toughness of the human dentin. The mid-sagittal sections of the dentin were subjected to Vickers hardness testing after removal of organic component from dentin by heat treatment. The heat treatment temperature for human dentin was determined as 800°C by thermogravimetric analysis in order to facilitate complete removal of organic component. The removal of proteins from dentin was confirmed by Fourier transform infrared spectroscopy. The significant difference in hardness and fracture toughness values was found among the heat treated and untreated dentin (p<0.001). This study confirms the role of organic component of dentin on mechanical properties. Thus, helps in research and development of novel reparative materials.
Keywords
Human dentin; collagen; hardness; fracture toughness; thermo gravimetric analysis; Fourier transform infrared spectroscopy; heat treatment
Download this article as:| Copy the following to cite this article: Tamilselvi R, Rajan R. M, Kumari V. A, Kandaswamy D. Efficacy of Organic Component on Hardness and Fracture Toughness of Human Dentin Using Heat treatment – In vitro Study. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Tamilselvi R, Rajan R. M, Kumari V. A, Kandaswamy D. Efficacy of Organic Component on Hardness and Fracture Toughness of Human Dentin Using Heat treatment – In vitro Study. Biomed Pharmacol J 2016;9(3).Available from: http://biomedpharmajournal.org/?p=11834 |
Introduction
The mature human dentin is composed of 70% of inorganic component, 20% of organic material and 10% of water by weight.1 The inorganic material is mainly composed of calcium phosphate related to the hexagonal hydroxyapatite, whose chemical formula is Ca10(PO4)6·2(OH).2 The organic matrix is composed of collagen (85-90%) and a variety of non collagenous proteins.3,4,5 Most of the collagen is type I.6 The dentin serves as an elastic foundation for the enamel and as a protective enclosure for the pulp and therefore mechanical properties are of utmost importance in determining the tooth strength. The most striking morphological feature of dentin is the tubule, with its hypermineralized peritubular cuff, influenced the mechanical properties of dentin.7 The elastic behavior of dentin was due to intertubular dentin matrix, and not the dentinal tubules.8 Any changes in the mineral imbalance caused by caries or developmental disorders, compromise the mechanical integrity of the tooth. But, the influence of organic components of dentin on the mechanical properties has not been clearly proved. Hence, the objective of the present study was to acquire the details on the influence of organic matrix on hardness and fracture toughness of the human dentin.
Methods
Sample preparation
Twenty human mandibular premolars free of caries extracted for orthodontic reasons from young individuals were used for this study. The premolars were rinsed with saline after extraction and stored at – 40°C and was used within one month. The samples were mounted in acrylic resin at room temperature and cut in the mid sagittal plane using hard tissue microtome (Leica, Rotterdam) to obtain specimens with the thickness of 1.5mm.The study included two groups, the control group being the samples with organic component (n=8). The experimental group was heat treated to remove the organic content in the dentin specimen (n=8).
Thermal treatment
The heat treatment temperature for human dentin was determined as 800°C by thermogravimetric analysis in order to facilitate complete removal of organic component (Fig. 1). The heat treatment was performed with 8 specimens in a tubular furnace (Indfur, India) with oxygen atmosphere for 4 hours to 800°C to remove the organic matrix from the dentin. The removal of proteins from dentin was confirmed by Fourier transform infrared spectroscopy (Perkin Elmer, USA) (Fig. 2). The untreated 8 samples were used as a negative control.
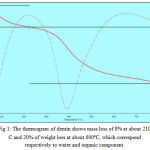 |
Figure 1: The thermogram of dentin shows mass loss of 8% at about 210°C and 20% of weight loss at about 800ºC, which correspond respectively to water and organic component.
|
 |
Figure 2: The differences in the ratios of peak intensities of amide I (major protein absorbance band) and PO4 were used to monitor organic matrix removal. The amide I absorbance at 1600-1700 cm-1 was significantly reduced in the heated sample, due to protein removal.
|
Hardness and fracture toughness analyses
The hardness of the dentin was evaluated using Vickers diamond tip. The tests were carried out at room temperature with different loads form 0.98N – 49N, until crack formation and dwell time of 15 seconds. For each sample, 8 indentations were made in the circumpulpal dentin. A distance of at least two times the impression diagonal was kept between the indentations to minimize interactions between neighboring indentations. The Vickers hardness was calculated according to the equation, HV = 1.854x P/ D2, where, HV -Vickers hardness, P – applied load, D – indentation diagonal (µm).9
The fracture toughness was computed according to the equation, KIC = P/ l 3/2 β0 ,where, KIC – Fracture toughness , P – load, l – crack length, β0 – Indenter constant equal to 7 for a vicker’s indenter.10
Results
In the mid sagittal plane, the lowest hardness values were observed in the heat treated specimens when they were subjected to different loads from 0.98N – 49N (Table, Fig. 3a). In both treated and untreated specimens, a pronounced load-dependent hardness behavior was evident. The hardness decreased as the load increased (Table). The decrease in hardness was observed up to 55% in the samples in which organic matrix was absent by heat treatment. The optical micrography revealed smaller indentation impression in the untreated specimens compared to the samples in which organic matrix were absent. The fracture toughness and hardness values were directly correlated to each other and inversely proportional to load (Table, Fig. 3). The fracture toughness is related to the crack length. The optical micrography of the samples revealed the crack formed at a greater load in untreated than heat treated specimens. The crack was formed in untreated dentin samples when the load of 49N was applied. The samples in which the organic matrix was removed, the crack formation occurred when subjected to the load of 9.8N (Table, Fig. 3).The removal of organic matter led to a substantial decrease in the fracture toughness of about 57% in the specimens.
Table 1: Mechanical characteristics of untreated and heat treated human coronal dentin.
| Load (N) | Mean
Hardness (Kg / mm2) |
Mean
Crack length (mm) |
Mean
Fracture toughness (Mpa m½) |
|||
|
Untreated
|
Heat treated | Untreated | Heat treated | Untreated | Heat treated | |
| 0.98 | 61.42 | 34.25 | ||||
| 1.96 | 60.06 | 31.76 | ||||
| 2.94 | 58.63 | 27.16 | ||||
| 4.9 | 56.25 | 23.68 | ||||
| 9.8 | 52.23 | 20.68 | 97.87 | 1.46 | ||
| 19.6 | 46.82 | 17.02 | ||||
| 29.4 | 40.66 | 15.37 | ||||
| 49 | 29.62 | 12.25 | 153.75 | 3.43 | ||
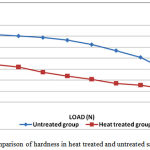 |
Figure 3a: Comparison of hardness in heat treated and untreated samples
|
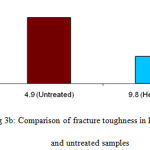 |
Figure 3b: Comparison of fracture toughness in heat treated and untreated samples
|
The Students t – test was used to test for significant difference in hardness and fracture toughness values among the heat treated and untreated dentin specimens. The statistically significant difference (p<0.001) was found between the control and experimental groups in the parameters, hardness and fracture toughness.
Discussion
Human dentin essentially is a hydrated composite composed of nanocrystalline carbonated (calcium – phosphate – based ) apatite mineral (45% by volume), type I collagen fibrils (33% by volume) and water (22% by volume).The results of this study demonstrated that, although the dentin comprises 20% of organic matter by weight, it significantly influenced its mechanical properties confirming previous hypothesis.11 With the objective to prove the validity of this hypothesis, we intended to remove the organic matrix in the dentin to check for its influence in hardness and fracture toughness. Thermal treatment method of organic removal was chosen since wet chemical techniques have been shown to alter the mineral content of bone which is similar to dentin.12 Heating at high temperature (700°C – 900°C) removed the organic constituents of cortical bone and the coralline hydroxyapatite without, apparently, affecting the interlocking framework of the hydroxyapatite crystallites.13,14,15
The results of the present study showed statistically significant difference (p<0.001) in the mechanical properties tested, between the control and experimental groups as the collagen fibrils in dentin are roughly 50 – 100 nm in diameter; they are randomly oriented in a plane perpendicular to the direction of dentin formation16 and the mineral occupies two sites within this collagen scaffold: intrafibrillar (inside the periodically spaced gap zones in the collagen fibril) and extrafibrillar (in the interstices between the fibrils). The apatite crystals are believed to nucleate initially in the gap zone, followed by secondary mineralization of the interstitial positions between the fibrils.17 The hydroxyapatite crystals, which average 0.1 µm in length, are formed along their fibers with their long axes oriented parallel to the collagen fibers.18 It is generally believed that collagen fibrils form a felt work structure laid down perpendicular to the tubules and in the plane of the advancing mineralization front19 and the intertubular dentin matrix, and not the dentinal tubules, dominates the elastic behavior.8 Hence, the removal of organic component in human dentin significantly decreased the hardness and fracture toughness and weakens entire structure of dentin, as the type I collagen which acts as a scaffold that accommodates a large proportion of mineral (56%) in holes and pores of fibrils.1
Conclusion
Our findings provide information that the removal of organic matrix resulted up to 55% and 57% decrease in hardness and fracture toughness, which implied that the organic matrix is not only essential during hard tissue formation, but also has a functional role in the mature tissue and emphasizing its importance in maintaining the integrity of human dentin.
References
- Ten cate AR. Oral histology: development,structure, and function 7th edn. St. Louis: Mosby, 2008.
- Le Geros RZ. Calcium phosphates in oral biology and medicine, Howard M. Myers, Edn, San Francisco, California, 1991.
- Veis A, Spector AR, Zamascianyk H. Isolation of an EDTA- soluble phosphoprotein from mineralizing bovine dentin. Bio chem Biophys Acta 1972;257:404-413.
- Termine JD, Kleinman HK, Whitson WS, Conn KM, McGarvey ML. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 1981;26:99-105.
- Price PA, Otsuka AS, Poser JW, Kristaponis J, Raman N. Characterization of a γ – carboxyglutamic acid – containing protein from bone. Proc Natl Acad Sci USA 1976;73:1447-1451.
- Maglorie, Joffre, Grimaud, Herbage, Couble, Chavrier. Identification of type I collagen fibrils in human dentine. Electron microscope immunotyping. Cell Mol Life Sci 1983;39:169-171.
- Sano H, Ciucchi B, Matthews WG, Pashley DH. Tensile properties of mineralized and demineralized human and bovine dentin. J Dent Res 1994;73:1205-1211.
- Kinney JH, Balooch M, Marshall GW, Marshall SJ. A micromechanics model of the elastic properties of human dentine. Arch Oral Biol 1999;44:813–822.
- ASTM. ASTM designation E 384. In: Standard test method for microhardness of materials. Philadelphia: American Society for Testing and Materials, 1991.
- Navamathavan R, Arivuoli D, Attolini G, Pelosi C, Chi Kyu Choi. Mechanical properties of some binary, ternary and quaternary III-V compound semiconductor alloys. Physica B 2007;392:51-57.
- Jameson MW, Hood JAA, Tidmarsh BG. The effects of dehydration and rehydration on some mechanical properties of human dentin. J Biomech 1993;26:1055-1065.
- Broz JJ, Simske SJ, Corley WD, Greenberg AR. Effects of deproteinization and ashing on site-specific properties of cortical bone. J Mater Sci: Mater Med 1997;8:395-401.
- Cowin. Bone mechanics. CRC Press, Boca Raton,FL,1980.
- Wang PE, Chaki TK. Sintering behaviour and mechanical properties of hydroxyapatite and dicalcium phosphate. J Mater Sci: Mater Med 1993;4:150-158.
- Murugan R, Panduranga Rao k and Sampath Kumar TS. Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties. Bull.Mater Sci 2003;26:523-528.
- Jones SJ, Boyde A. Ultrastructure of dentin and dentinogenesis. In: Dentin and dentinogenesis. Linde J, editor. Boca Raton: CRC Press,1984,81-134.
- Landis WJ, Librizzi JJ, Dunn MG, Silver FH. A study of the relationship between mineral content and mechanical properties of turkey gastrocnemius tendon. J Bone Mineral Res 1995;10:859–867.
- Orban. Oral histology and embryology 11th edn. St. Louis: Mosby, 2001.
- Johnson NW, Poole DFG . Orientation of collagen fibers in dentine. Nature 1967;213:695–696.








