Manuscript accepted on :September 02, 2016
Published online on: --
Plagiarism Check: Yes
Ni Wayan Bogoriani and I. Wayan Sudiarta
Chemistry Departement, Faculty of Mathemathic and Natural Science, University of Udayana, Denpasar, Bali, Indonesia.
Correspondent Author E-mail: bogi_wayan@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1028
Abstract
This study was conducted over 10 weeks using several types of oils containing unsaturated fatty acids, saturated fatty acids and trans fatty acids, namely coconut oil, bulk oil, used cooking oil and pig. The purpose of this study is to determine the effects of used cooking oil of the sress oxidative and inflammation on wistar rat. The study design was a randomized post-test only control group design, conducted in male wistar rats. Twenty-five rats were divided into five groups: control, treatment-1, treatment-2, treatment-3 and treatment-4, each of 5 rats. The control group were given only the standard diet, treatment-1 were given standard food and pig oil, treatment-2 were given standard food and used cooking oil, treatment-3 were given standard food and bulk oil and the treatment-4 were given standard food and coconut oil. The allocation of this fatty diet conducted by taking into account all sampel shows that the fatty-high diet is 2.5 mL/day. After 10 weeks of treatment for blood plasma samples were taken for examination of stress oxidative (MDA) and Inflammation (TNF-α and IL-6). The average different of content among the groups is tested using One Way ANOVA method, which is then followed by LSD, in which the statistical testing is declared significant if p < 0.05. The results showed an increase in the levels of MDA, TNF-α and IL-6 blood plasma were significantly (p <0.05) in all treatments compared to the control. However, the group treated with coconut oil gave levels of MDA and TNF-α were not significantly different (p> 0.05) and was lower than the control. Two treatment groups showed differences in the levels of MDA, TNF-α and IL-6 the highest is the treatment used cooking oils. The results showed an increase in significantly (p <0.05) in the treatment of used cooking oil compared with the control and other treatments (coconut oil, oil bulk oil and pig). Based on these results it can be concluded that the used cooking oil has the potential to cause oxidative stress and inflammation which is a risk factor for atherosclerosis formation.
Keywords
used cooking oil; trans fatty acids; coconut oil; bulk oil; pig oil; TNF-α; MDA; IL-6
Download this article as:| Copy the following to cite this article: Bogoriani N. W, Sudiarta I. W. Effect of Used Cooking Oil of the Stress Oxidative and Inflammation on Wistar Rats. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Bogoriani N. W, Sudiarta I. W. Effect of Used Cooking Oil of the Stress Oxidative and Inflammation on Wistar Rats. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=9155 |
Introduction
Atherosclerosis is the hardening and thickening of the walls of the arteries that occurs due to the deposition of fat, complex carbohydrates and blood products, connective tissue and calcium. Atherosclerosis is a major cause of coronary heart disease (Scott M. Grundy et al.,2002). One of the major risk factors for atherosclerosis is dyslipidemia. Dyslipidemia is a disorder of blood fat fraction, which is characterized by elevated levels of LDL and HDL levels decrease. If cholesterol in the liver, indicating excessive cholesterol levels, it can interfere the metabolism so that cholesterol accumulate in the liver. If this situation is left for a long time, then the excess cholesterol will stick to blood vessel walls and cause atherosclerosis plaque. Atherosclerotic plaque formation involves inflammation process as an initiator and propagator (speed up the process of the formation of atherosclerosis). Evidence of the involvement of the inflammatory process of the formation of atherosclerotic plaque is the discovery that an increase in inflammatory markers interleukin-6 and tumor necrosis factor alpha in patients with cardiovascular disease. These inflammatory factors can affect the occurrence of endothelial dysfunction, the condition of hypercholesterolemia-induced expenditure by macrophages (foam cells) due to ox-LDL produces a state of oxidative stress (Scott M. Grundy, et al., 2002; Subowo; 2009). In the study found besides the presence of fat in the arteries undergo atherosclerosis, also found various types of cells involved in chronic inflammatory processes in blood vessels, with a variety of complications and clinical complaints (Phipp, 2000; Arenillas et al., 2008). Crucial immune mediators involved in the process of atherosclerosis is a ligand CD 40 which is a potent activator in the pathogenesis of atherosclerosis. CD 40 ligand binds to the receptor CD 40 which is a member of the TNF receptor expressed on endothelial initiates the inflammation and atherosclerosis through increased production of proinflammatory interleukin (Phipp, 2000; Xia et al., 2007).
Oxidative stress is a condition in which the amount of free radicals in the body exceeds the capacity of antioxidants in the body so that the body can not neutralize them. As a result, the intensity of the oxidation process of normal body cells becomes higher and cause more severe damage (West, 2000). Levels of free radicals exceeds of the antioxidant produced will cause imbalance resulting in oxidative stress. Dyslipidemia may increase oxidative stress (Ceriello et al., 1993; Giugliano et al., 1995; West, 2000) and reduced antioxidant defenses (Antoniades et al., 2003; Stocker and Keaney, 2004; Penckofer et al., 2002). Increased oxidative stress contributes to vascular disorders, inflammatory function, atherosclerosis thrombosis, and eventually cause disease of blood vascular (Giugliano, 2000). Malondialdehyde (MDA) unstable compounds from the decomposition of lipid peroxide as a result of reactions between free radicals with unsaturated fatty acids and is one of the parameters of oxidative stress in the body.
Fatty acid especially as ester the natural oils and fats but there can be in the form of free fatty acids esterified as that is a form transfor contained in plasma. Fatty acids contained in natural fats usually are straight-chain derivatives containing an even number of carbon atoms. The chain can be saturated (containing no double bonds) or unsaturated (containing one or more double bonds). Effect of dietary saturated fatty acids and cholesterol may increase serum cholesterol can lead to atherosclerosis which is a major factor in the occurrence of premature cardiovascular disease (Winarno, 1986; Djamilah-Najm, 2013).
Oils containing unsaturated fatty acids plural (Polyunsaturated Fatty Acid / PUFA) purported to lower blood cholesterol and increase the value of other health. However, if used for frying repeatedly, the unsaturated fatty acids (either from the fryer oil and from fried foods) will be transformed into fatty acids “Trans”, peroxides group and other free radical compounds that can stimulate the occurrence of malignancy. While oil containing saturated fatty acids (Saturated Fatty Acid / SFA) are better able to withstand the heat and will not turn into trans fatty acids and other harmful compounds (Silllahi J., 2000).
Trans fatty acids not only increase LDL cholesterol levels, but simultaneously also reduce levels of HDL cholesterol (Enig, 2004). High levels of total cholesterol in the blood plasma, LDL cholesterol, VLDL cholesterol and low HDL cholesterol is associated with coronary atherosclerosis in adults (URL: //WWW.mercola. Com / 2000 / June / 10 / trans_fats.htm). Recent data from the Women’s Health Study showed that the use of the ratio of total cholesterol / HDL cholesterol is a powerful predictor of risk more than LDL cholesterol by only just (URL: //WWW.coconutoil.com/John%20 Kabara.pdf).
In order to reveal more about the effects of various oils on cholesterol metabolism in the body, has conducted research on the effects of various oil against oxidative stress and inflammation in male Wistar rats. Oil used as research material, because the oil contains a lot of saturated fatty acids, unsaturated and unsaturated fatty acids which have been transformed into trans fatty acids that are dangerous to health.
Material and Methods
Place and Research Time
Research has been conducted at the Center study of Animal Diseases (CSAD) Faculty of Veterinary Medicine Udayana University. Analysis of MDA, TNF-α and IL-6 in Analytical Laboratory Unit conducted at Udayana University. The study was conducted for 4 months (preparation, maintenance of rats, blood plasma analysis).
Material and Equipment Research
Research materials are various types of oils (coconut oil, bulk oil, used cooking oils and lard). To determine the effect on the metabolism of cholesterol, then each oil was given to the rats. A total of 25 Wistar rats are male and white, with 2.5 months of age and initial body weight of 150-200 g, were used in this study. The main tools used are the rat cage, EDTA blood tubes to obtain plasma, while for the analysis of blood samples used several tools, among others micropipette Socorax size 10 (Swiss Madle), Spectrophotometer (Hitachi Japan) and 100 ml test tube.
The main ingredients for this study is coconut oil, bulk oil, used cooking oil, lard, standard food pellets CP 552 (as a control), and drinking water. Materials for analysis of MDA, TNF-α and IL-6 blood plasma of rats that reagent standard solution kit rat MDA, ab100772-IL-6 rat ELISA kit pg / mL, BMS622 / BMS622TWO / BMS622TEN kit rat TNF-α platinum ELISA.
Research Procedure
Rats Maintenance
A total of 25 experimental rats aged 2.5 months, maintained for 10 weeks. Adaptation is done during the week prior to the feed given treatment. Feed and water provided ad libitum. Twenty-five rats were divided into five groups: control, treatment_1, treatment_2, treatment_3 and treatment_4, respectively 5 rats. The control group was only given a standard diet, treatment_1 given standard food and pig oil, treatment_2 were given standard food and used cooking oil, treatment_3 were given standard food and bulk and treatment_4 were given standard food and coconut. Each treatment was given a high-fat diet at 2.5 ml / day. After 10 weeks of treatment for blood plasma samples taken for examination MDA, TNF-α and IL-6.
Withdrawal of Rats blood
After 10 weeks of treatment the rats were fasted 14 hours, and then blood is drawn through the eye orbital. Before blood sampling, rats anesthetized with ether solution. 3 ml of blood collected and centrifuged at 3500 rpm 5 minutes.
Analysis of MDA, TNF-α and IL-6 in Blood Samples
The level of MDA was performed using a method based on the amount of malondialdehyde Espinosa Mansila which react with acids tribarbiturat in units of nmol / mL.
The level of TNF-α and IL-6 plasma performed by ELISA, with units of pg / mL.
Statistical analysis
Statistical analysis was performed with the statistical analysis system. Values are expressed as mean ± SD. Results were analyzed by one-way ANOVA, and differences among the treatments were determined by least-significant-difference test (LSD). Alpa 0.05 was used to determine statistically significant differences
Results and Discussion
Results
Determination of Levels of MDA, TNF-α and IL-6 Rat Blood Plasma
Wistar rats were given treatment for 10 weeks and the last day of the study the rats were fasted for 14 hours to pull all the food and drink of the cage, then blood is taken and evaluated according to the study protocol. Comparison of the mean levels of MDA, TNF-α and IL-6 levels are presented in Table 1 and Figure 1.
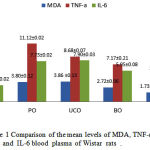 |
Figure 1: Comparison of the mean levels of MDA, TNF-α, and IL-6 blood plasma of Wistar rats. |
Table 1: The mean levels of MDA, TNF-α dan IL-6 blood plasma of wistar rats
| Parameter | control | P | PO | p | UCO | P | BO | p | C | P |
| MDA (nmol/mL) | 1,90±0,23bcd | 0,000
0,000 0,000 0,099
|
3,80±0,12ade | 0,000
0,535 0,000 0,000 |
3,86±0,14ade | 0,000
0,535 0,000 0,000 |
2,72±0,06abce | 0,000
0,000 0,000 0,000 |
1,73±0,18bcd | 0,099
0,000 0,000 0,000 |
| TNF-α (pg/mL) | 5,86±0,13bcde | 0,000
0,000 0,000 0,000 |
11,12±0,02acde | 0,000
0,000 0,000 0,000 |
8,68±0,07abde | 0,000
0,000 0,000 0,000 |
7,17±0,21abce | 0,000
0,000 0,000 0,000 |
5,26±0,08abcd | 0,000
0,000 0,000 0,000 |
| IL-6 (pg/mL) | 3,19±0,02bcde | 0,000
0,000 0,000 0,000 |
7,73±0,02acde | 0,000
0,000 0,000 0,000 |
7,90±0,03abde | 0,000
0,000 0,000 0,000 |
5,95±0,08abce | 0,000
0,000 0,000 0,000 |
4,56±0,07abcd | 0,000
0,000 0,000 0,000 |
Mean ± SD (n = 6) followed by different letters (superscript) in the same row indicate significant differences; MDA : Malondialdehyde; TNF-α : Tumor necrosis factor-α; IL-6 : Interleukin-6; PO: pig oil ; UCO: used cooking oil; BO: bulk oil; CO :coconut oil
a Represents significant difference from controlp<0,05
b Represents significant difference from PO p<0,05
c Represents significant difference from MJ p<0,05
d Represents significant difference from BO p<0,05
e Represents significant difference from CO p<0,05
a Represents significant difference from control at p < 0.05.
bRepresents significant difference from high cholesterol at p < 0.05.
Based on the data in Table 1 above it can be seen that an increase in the mean levels of MDA, TNF-α, IL-6 in all treatment groups (Figure 1.) with a significant difference (p <0.05) in the appeal control. The treatment_1 with pig oil: 3.80 ± 0.12 pg / mL (MDA); 11.12 ± 0.02 pg / mL (TNF-α); 7.73 ± 0.02 pg / mL (IL-6); in treatment_2 with used cooking oil: 3.86 ± 0.14 pg / mL (MDA); 8.68 ± 0.07 pg / mL (TNF-α); 7.90 ± 0.03 pg / mL (IL-6); the treatment_3 with bulk oil: 2.72 ± 0.06 pg / mL (MDA); 7.17 ± 0.21 (TNF-α); 5.95 ± 0.08 pg / mL (IL-6); treatment_4 with coconut oil: 1.73 ± 0.18 pg / mL (MDA); 5.26 ± 0.08 pg / mL (TNF-α); 4.56 ± 0,07pg / mL (IL-6); and as controls: 1.90 ± 0.23 pg / mL (MDA); 5.86 ± 0.13 pg / mL (TNF-α); 3.19 ± 0.02 pg / mL. But there was no significant difference in the treatment with coconut oil on MDA and TNF-α compared to controls (p> 0.05). Treatment with used cooking oil showed levels of the highest in the MDA and IL-6, followed by pig oil, bulk oil, control oil and coconut on inspection MDA, while the examination of IL-6, after the used cooking oil is followed by pig oil, bulk oil, coconut oil and control. On examination of TNF-α showed that the highest pig oil, and used cooking oil, bulk oil, and coconut oil and control.
Discussion
Based on the data in Table 1 it can be seen that after 10 weeks of treatment, the effect of fatty acids of various atherogenic diet has increased in all treatments compared to controls at MDA, TNF-α and IL-6 with a significant difference (p <0.05). However, treatment with coconut oil does not occur a significant difference (p> 0.05) on the examination of MDA and TNF-α. Increased levels of MDA and IL-6 is the highest in the treatment group were given used cooking oil, followed by the group treated with pig oil, bulk oil, control, and coconut oil for MDA examination. So treatment with coconut oil, the lowest levels of MDA. Although the examination of the IL-6 treatment with coconut oil showed significant differences compared to the control.
Based on the literature that coconut oil contains medium chain saturated fatty acid and about 64% lauric acid (C12) 47-53% (Bawalan and Chapman, 2006). Low linolenic acid content of about 0.90 to 1.72% (Marina, et al., 2009). Coconut oil also contains compounds called polyphenols and has an effect as antimocrobe, anti-inflammatory and emollient to the skin of patients with atopic dermatitis (Verallo-Rowell, et al., 2008). Coconut oil (VCO) is useful in lowering the fat component when compared with copra oil, reducing total cholesterol, triglycerides, phospholipids, LDL and VLDL and increasing HDL cholesterol in serum and tissue. The content of polyphenols can prevent the oxidation of LDL by reducing the formation of carbonyl groups (Navin and Rajamohan, 2004). Coconut oil (VCO) has the strongest scavenging effect on 1,1-diphenyl-2-picrylhydrazyl and the highest antioxidant activity based methods of beta-carotene-linoleic bleaching and has the highest power through chilling methods. Phenolic compounds owned coconut oil is very meaningful change production of cytokines or antioxidants, resulting in barriers to the production of IL-6 and IL-8 (Gaulliard, et al., 2008). Phenol antioxidants potently inhibit signal which induces TNF-α and its target gene IL-1β and IL-6 in macrophages cells. Increased oxidative stress on a diet high in long-chain saturated fatty acids that have the highest composition of palmitic acid appears in the delivery of lard.
In this study are presented in Table 1 show that treatment with used cooking oil on inspection MDA, TNF-α and IL-6 occurs a significant difference (p <0.05) compared with controls, whereas treatment with coconut oil makes a difference were not significant (p> 0.05). This is probably caused by a triglyceride composition of saturated vegetable fats differ from saturated fat in pigs. In the triglyceride composition of vegetable fats are often found in saturated-unsaturated-unsaturated and saturated-unsaturated-unsaturated. In the reverse situation lard, which is usually saturated-unsaturated-unsaturated and unsaturated-saturated-unsaturated (Winarno, 1986).
Increased plasma MDA of rats treated with used cooking oil, pig oil and bulk oil is a sign of oxidative stress due to increased total cholesterol (Bogoriani and Ratnayani, 2015) and hypercholesterolemia (Singhania et al., 2008; Abdelhalim, 2010). Increase in total plasma cholesterol will cause an increase in ROS through NADPHA oxidase enzyme activity (Cai and Harrison, 2000; Madamanchi et al., 2004). Increased ROS, especially superoxide ions will cause lipid peroxidation resulting in increased MDA. The results are consistent with studies in rabbits given a diet high in cholesterol, an increase in total cholesterol and MDA were significantly (Abdelhalim, 2010). Research in mice were given a high-fat diet also led to an increase in MDA, resulting in hypercholesterolemia in these mice (Han et al., 2007; Chen et al., 2011). According to research Vogel et al., 1997 found that foods high in saturated fatty acids and trans fatty acids may be an increase in oxidative stress and the production of inflammation. Lard contains higher saturated fatty acids that are more easily absorbed in the intestine and used cooking oils containing trans unsaturated fatty acids (Silalahi, J. 2000), both of which can increase the possibility of oxidative stress and inflammation. Based on the literature that has not been used cooking oil which contains saturated fatty acids and unsaturated. Unsaturated fatty acids are recognized plural can lower blood cholesterol and increase the value of other health. However, if used for frying repeatedly called cooking oil, the unsaturated fatty acids will be changed into fatty acids “Trans”, group peroxides and other free radical compounds will stimulate malignancy. Unsaturation degree oil temperature increases will shrink the same time so that the fatty acid chains break up into free radicals that increase blood cholesterol higher and higher so that the risk to health (Tuminah; S. 2009; Admin, 2013, Bogoriani and Ratnayani, 2015). While oil containing saturated fatty acids are better able to withstand the heat and will not turn into trans fatty acids and other harmful compounds (Mozaffarian, et al., 2006; Tuminah, S., (2009).
The provision of a high-fat diet mainly from used cooking oils and lard can increase plasma total cholesterol (Bogoriani and Ratnayani, 2015), which allows to form oxLDL as a result of oxidative stress (Bahorun et al., 2006). OxLDL formed contains a variety of bioactive molecules such as oxysterols, phospholipids and fatty acid peroxide, which will increase the expression of VCAM-1 on endothelial (Naeto et al., 2004). Increased expression of VCAM-1 also increases the proinflammatory cytokines that the early stages of atherosclerosis of blood vessels (Naeto et al., 2004). So an increase in plasma cholesterol will increase oxidative stress (MDA) and inflammatory cytokines (TNF-α and IL-6), so there is a positive correlation between plasma cholesterol levels with oxidative stress and inflammation (Gustavsson et al., 2010).
In this study, coconut oil gives plasma cholesterol levels with a significant difference (p> 0.05) compared to controls (Bogoriani and Ratnayani, 2015) and provide the levels of MDA, TNF-α and IL-6 the lowest compared to controls. It is proved that coconut oil is useful as an antioxidant and anti-inflammatory. Coconut oil contains medium chain saturated fatty acids, unsaturated fatty acids and polyphenolic compounds that can act as antioxidants, either directly or indirectly. Polyphenol compounds found in plants has proven to be an antioxidant, which protects the vascular endothelium, anti-inflammatory and can be as immunomodulators (Wang and Mazza, 2002), as well as to inhibit atherosclerosis through several mechanisms (Xu et al., 2004). Unsaturated fatty acids such as omega-3 can prevent oxidative stress is believed to cause the various types of disorders, one of which is endothelial dysfunction, causing atherosclerosis. The link between atherosclerosis, oxidative stress and inflammation have been studied for a long time and shown to be beneficial to health (Prior, 2003; Herring and Albrecht, 2005). Whereas lard and cooking oil gives MDA, TNF-α and IL-6 is higher than the control, thereby potentially causing atherosclerosis. Atherosclerosis is the leading cause of death by almost 50% of all deaths in developing countries. According to WHO figures were expected to rise as a cause of illness in 2010 (Frolov and Hui, 2007).
Conclusion
The results showed that there were significant differences (p <0.05) in the levels of MDA, TNF-α and IL-6 blood plasma of Wistar rats were fed a diet of various types of fine oil bulk oil, used cooking oils and lard, every day is adlibitum compared to controls, but on coconut oil treatment does not occur a significant difference (p> 0.05).
The increase in the levels of MDA, TNF-α and IL-6 in the blood plasma of rats fed a diet of used cooking oils and lard higher when compared to control and other treatments (MK, and MC), so we need to watch out for use. Increase in total cholesterol in the blood of mice with the treatment of used cooking oil, pork oil and bulk oil turned out to increase oxidative stress correlates with increased inflammation, especially used cooking oil and lard possibilities tend to atherosclerosis which is a risk factor for coronary heart disease.
Suggestion
Need further research using the technique imunohistopatologi to determine directly the degree of endothelial damage caused by the response of oxidative stress and inflammation during 10 weeks of treatment by providing a diet of various oils mainly used cooking oil in mice, to be seen more clearly the negative impact of cooking oil contains fatty acids saturated fatty acids “trans” that are dangerous to health.
Acknowledgements
The assistance provided during the study, the authors would like to thank all members of the researcher. Also for the Udayana University on BOPTN funding for this research.
References
- Abdelhalim, M.A.K. The Potential Influence of High Cholesterol Diet-induced Oxidative stress on Composition and Properties of Red Blood Cells in Rabbit. African Journal of Microbiology Research, 2010; 4(9): 836-843.
- Admin. Kenapa Minyak Jelantah Itu Berbahaya? http://ikiopo.com/kenapa-minyak-jelantah-itu-berbahaya, 2010.
- Antoniades C, Tousoulis D, Tentolouris C, Toutouzas P, Stefanadis C. Oxidative stress, antioxidant vitamins, and atherosclerosis. From basic research to clinical practice. Herz, 2003; 28: 628–638. Find this article online
- Arenillas, J. F., Alvares-Sabin, J., Molina, C. A. Progression of Symptomatic Intracranial Large Artery Atherosclrosis is Associated with a Proinflammatory State and Impaired Fibrinolysis. Stroke, 2008; 39:1456-62.
- Bawalan, D.D., Chapman, K.R. Virgin Coconut Oil Production Manual for Micro-and Village-Scale Processing. Edisi pertama. Bangkok : Thammada Press Co Ltd. 2006; P. 15.
- Bogoriani,N.W., dan Ratnayani,K. Efek Berbagai Minyak pada Metabolisme Kolesterol terhadap Tikus Wistar. Jurnal Kimia, 2015; 9(1) :53-60.
- Bahorun, T., Soobratte, M.A., Luximon-Ramma, V., Aruoma, O.I. Free Radicals and Antioxidants in Cardiovascular Health and Disease. Internet Journal of Medical Update, 2006; 1 (2):25-41.
- Cai, H., Harrison, D. G. Endothelial Dysfunction in Cardiovascular diseases: The Role of Oxidant Stress. Circulation Research, 2000; 87:840-4
- Ceriello A, Quatraro A, Giugliano D. Diabetes mellitus and hypertension: the possible role of hyperglycaemia through oxidative stress. Diabetologia, 1993; 36: 265–266. Find this article online
- Chen, W. P., Mao, T. J., Fan, L., Zhou, Y. H., Yu, J., Jin, Y., Hou, P. C. Effect of purple sweet potato on lipid metabolism and oxidative stress in hyperlidemic rats. Chinese, 2011; 40(4):360-4
- Chen S-J, Yen C-H, Huang Y-C, Lee B-J, Hsia S. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. PLoS ONE, 2012; 7(9): e45693. doi:10.1371/journal.pone.0045693.
- Djamilah-Najmuddin http://www.djamilah-najmuddin.com/antara-minyak-jelantah-stroke-dan-kanker. Maret 12th, 2013.
- Enig MG. Coconut : In Support of Good Health in the 21st Century. (Cited 2004 Oct.28). Available from :URL://www.livecoconutoil.com/maryenig.htm Nutrition, [cited 2003 Sept 19]. Available from:URL://www. 1 stholistic.com/Nutrition/hol_nutr_fat.htm
- Frolov, A., and Hui, D. Y. The Modern Art of Atherosclerosis, Thrombosis, and Vasculer
- Gaulliard, B., Grieve, D., Wilson, R., Crozier, R., Jenkins, C., Mullen, W.D., Lean, M. The effect of dietary phenolic compound on cytokine and antioxidant production by A549 Cells. J Med Food, 11: 382-4 Gaulliard, B., Grieve, D., Wilson, R., Crozier, R., Jenkins, C., Mullen, W.D., Lean, M.2008. The effect of dietary phenolic compound on cytokine and antioxidant production by A549 Cells. J Med Food, 2008;11: 382-4
- Gustavsson, C., Carl-David, A., Zetterqvist, A. V., Nilsson, J., Agardh, E., and Gomesz, M.F.. Vascular Cellular Adhesion Molecule-1 (VCAM-1) Expression in Mice Retinal Vessels is Affected by Both Hyperglycemia and Hyperlipidemia. PLOS One, 2010; 5(9): 12699-126100.
- Han, X., Shen, T., and Lou, H. Dietary polyphenol and their biological significance. Int. J. Mol. Sci, 2007; 8 : 950-988.
- Han, K. H., Matsumoto, A., Shimada, K., Sekikawa, M., Fukushima, M. Effects of anthocyanin-rich purple potato flakes on antioxidant status in F344 rats fed a cholesterol-rich diet. Br. J. Nutr. 2007;98(5):914-21.
- Health Risks from Processed Foods and the Dangers of Trans Fats, [cited2003Oct].Availablefrom:URL://www.mercola.com/2000/June/10/trans_fats.htm
- Health Oils from the Tree of Life (Nutritional and Health Aspects of Coconut Oil), [cited 2004 Jan]. Available from :URL://www.coconutoil.com/John%20Kabara.pdf.
- Herrmann, J., Saguner, A.M., Versari, D., Peterson, T.E., Chade, A., Olson, M., Lerman, L.O., Lerman, A. Chronic proteosome inhibition contributes to coronary atherosclerosis.
- Herring, T., and Albrecht, J. Functional foods. Extension circular. University of Nebraska. 2005.www.reeis.usda.gov/web/crisprojectpages/0196754.
- Madamanchi, N.R.,Vendrov, A., Runge, M.S.. Oxidative stress and vascular disease. Arterioscler Throm Vasc Biol, 2004; 25(1):29-38.
- Marina, A.M., Man, Y.B., Nazimah, S.A., Amin, I. Antioksidant Capacity and phenolic acids of virgin coconut oil. Int J.Food Sci Nutr. 2009; 60 (Suppl 2) : 114-123.
- Mozaffarian D, Katan MB, Ascherio A,Stampfer MJ, Willet WC. Trans fatty Acids and Cardiovascular Disease. The N Engl J.Med. 2006; 354:1601-1613.
- Naito, Y., Shimozawa, M., Manabe, H., Kuroda, M., Tomatsuri, N., Uchiyama, K., Takagi, T., Yoshida, N., and Yoshikawa, T..inhibitory effects of rad wine extracts on endothelial-Dependent adhesive interactions with monocytes induced by oxysterols. Biol. Res, 2004; 37:231-8.
- Nevin, K.G., and Rajamohan, T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL Oxidation. Clinical Biochemistry, 2004.;37:830-35.
- Prior, R.L. Fruits and Vegetables in the prevention of cellular oxidative damage. American Journal of Clinical Nutrition, 2003;78(30: 570-8.
- Silalahi J. Hypocholesterolemic Factors in Food : A Review. Indonesian Food and Nutrition Progress.. 2000; 7(1) : 26—35.
- Singhania, N., Puri, D., Madhu, S. V., and Sharma, S. B. Assessment of oxidative stress and endothelial dysfunction in Asian Indians with type 2 diabetes mellitus with and without macroangiopathy. QJM, 2008;101 (6):449-55.
- Sitepoe, M., Kolesterolfobia, Keterkaitannya dengan penyakit jantung. PT Gramedia Pustaka Utama. Jakarta, 1992.
- Stangl, K. dan Stangl, V. . The ubiquitin-proteasome pathway and endothel (Dys) function. Cardiovascular Research, 2010; 85(2): 281-290
- Stocker R, Keaney JF. Role of oxidative modification in atherosclerosis. Physiol, 2004; 84: 1381–1478. Find this article online.
- Scott M. Grundy, M.D., Diane Becker, Sc.D., Luther T., Clark, M.D., Richard S. Cooper, M.D., Margo A. Denke, M.D., Wm. James Howard, M.D., Donald B. Hunninghake, M.D., D. Roger Illingworth, M.D., Ph.D., Russell V. Luepker, M.D., M.S., Patrick McBride, M.D., M.P.H., James M. McKenney, Pharm.D., Richard C. Pasternak, M.D., F.A.C.C., Neil J. Stone, M.D., Linda Van Horn, Ph.D., R.D. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).Circulation Journal of the American Heart Association: 2002;106:3143.
- Subowo, Imunobiologi Edisi 2., CV Sagung Seto Jakarta,2009.
- Taniyama, Y and Griendling, K.K. Reactive oksygen species in the vasculature. Molecular and cellular mechanisms. Hypertention, 2003;42:1075-1081.
- Tuminah, S.,2009. Artikel Efek Asam Lemak Jenuh Dan Asam Lemak Tak Jenuh “Trans” Terhadap Kesehatan. Media Peneliti dan Pengembang. Kesehatan. Volume XIX, Suplemen IIPuslitbang Biomedis dan Farmasi: S13-S20.
- Verallo-Rowell, V.M, Dillgue, K.M., Syah-Tjundawan, B.S. Novel Antibacterial and emollient effects of coconut and virgin olive oil in Adult atopic dermatitis . Dermatitis, 2008; 19(6): 308-15.
- Wang, J., and Mazza, G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alfa in LPS/IFN-Gama-Activated RAW 264.7 Macrophages. J. Agric. Food Chem, 2002; 50(15): 4183-4189.
- Winarno, F.G. Kimia Pangan dan Gizi.Penerbit PT Gramedia, Jakarta. 1986.
- Xia, M., Zhu, H., Wang, Q., Ma, J., Hou, M., Tang, Z., Li, L., Ye, Q. Anthocyanin prevents CD-40-Activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arteriosclerosis, Thrombosis, and Vascular Biology, 2007; 27;519-24.
- Xu, J. W., Ikeda, K., Yamori, Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004;, 44:217-222.







