Malarvizhi Dakshinamoorthy1, Monisha Subramanian1, Kesavaram Padmavathi2, Krishnan Mahalakshmi2, Karthick Arumugam1 and Vivekanandhan Paramasivam1
1Department of Conservative Dentistry and Endodontics,Sree Balaji Dental College and Hospital, Bharath University, Chennai, Tamilnadu, India.
2Department of Microbiology, Sree Balaji Dental College and Hospital, Bharath University, Chennai, Tamilnadu, India.
Corresponding Author E-mail: drmalarendo@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1051
Abstract
This study is aimed to evaluate the effect of chocolate containing probiotic organism on the growth of Streptococcus mutans. Lactobacillus fermentum ,Lactobacillus delbrueckii, Bifidobacterium bifidum and Bifidobacterium longum were the probiotic organisms used in the study and dark chocolate was used as carrier. Dark chocolate was sterilized and the probiotics were incorporated in the dark chocolate according to the WHO/FDA guidelines. The probiotic organisms were incorporated in dark chocolate singly as well as in combination and was divided into seven groups. For comparison of the efficacy of the probiotic chocolate combinations, probiotic combinations (without chocolate) was assessed by agar well diffusion technique and the diameter of the zone of inhibition (in mm) around the wells were recorded. The antibacterial efficacy of the probiotic formulations (with and without chocolate) were compared to evaluate whether the chocolate could / could not alter the efficacy of the formulations. Sterile plain chocolate was used as control. The assay was performed in triplicates. The highest inhibitory effect was depicted by Bifidobacterium Longum when used alone and with probiotic chocolate. Lactobacillus fermentum, Bifidobacterium longum and Bifidobacterium bifidum proved to be best than all the probiotics in the study either alone or in combination. In the present study the least effect was shown by the probiotic chocolate with only Lactobacillus delbrueckii. All the probiotics used in our study had inhibitory effect on Streptococcus mutans and was found to be high in the presence of chocolate.
Keywords
Bifidobacterium; Lactobacillus; probiotics; Streptococcus mutans
Download this article as:| Copy the following to cite this article: Dakshinamoorthy M, Subramanian M, Padmavathi K, Mahalakshmi K, Arumugam K, Paramasivam V. Effect of Probiotic Chocolate in the Reduction of Streptococcus Mutans Count. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Dakshinamoorthy M, Subramanian M, Padmavathi K, Mahalakshmi K, Arumugam K, Paramasivam V. Effect of Probiotic Chocolate in the Reduction of Streptococcus Mutans Count. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11854 |
Introduction
Dental caries can be defined as destruction of the tissues of the tooth by bacterial fermentation of dietary carbohydrates. Streptococcus mutans plays a major role in dental caries, metabolizing sucrose to lactic acid1 using the enzyme, glucansucrase2. Acidogenicity and aciduric nature are the common features of caries-promoting bacteria. S.mutans is considered to be part of the “normal” flora of the human mouth. They are gram-positive cocci, which grow at temperatures between 18-40 degrees Celsius.
Sucrose is the only sugar that S. mutans can use to form glucan, a sticky polysaccharide3. It has the ability to catabolize carbohydrates and to produce acids. It has the ability to survive in low pH4. The concept of using probiotics is a novel step in combating dental caries.
Probiotics are defined as: “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host” (WHO 2001)5. Lilly and Stillwell in 1965 described Probiotics as “substances secreted by one microorganism, which stimulates the growth of another” and thus was contrasted with the term antibiotic 6. Though numerous probiotic strains exist, the most common bacteria used as probiotics are lactobacilli and bifidobacteria. Prebiotics are generally defined as not digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already established in colon, and thus, in effect, improve host health. The term Synbiotic refers to a combination of probiotics and prebiotics
In literature, there are very few studies that have evaluated the effect of probiotic chocolate on streptococcus mutans. Therefore, the purpose of this study is to investigate the antagonistic effect of selected probiotic organism such as Lactobacillus fermentum, Lactobacillus delbrueckii, Bifidobacterium bifidum and Bifidobacterium longum against Streptococcus mutans, the important cariogenic agent with chocolate as a carrier for probiotic. The null hypothesis is that the probiotic chocolate does not have any effect on Streptococcus mutans.
Materials and Methods
Lyophilized cultures of Lactobacillus fermentum MTCC9748, L. delbrueckii sub species lactis MTCC91, a standard strain of S. mutans MTCC497 were obtained from the Microbial Type Culture Collection and Gene bank (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. Pure cultures of Bifidobacterium bifidum, B. longum were obtained from the Department of Dairy Science, Madras Veterinary College, TANUVAS, Chennai. Fresh broth cultures of the Lactobacillus species were prepared in MRS broth, while the Bifidobacterium species and S. mutans were prepared in BHIB supplemented with sterile human serum, and the bacterial cell density was adjusted to 1 × 108 CFU/mL corresponding to 0.5 Mcfarland scale. Overnight cultures were used for the study, as bacteria exhibits maximum activity during log phase, the stage of rapid multiplication against S.mutans.
Sterilization of dark chocolate
Dark chocolate (Morde Foods Pvt Ltd, Maharastra, India) was sterilized by tyndallization with slight modifications7 to remove the vegetative bacteria and their spores, if any. Chocolate was heated in a water bath at 100˚ C for 3 hours and then stored at 37˚C for 24 hours. The process was repeated for three consecutive days. The heated chocolate was cultured on MRS agar, Columbia agar supplemented with 5% blood and Eosin methylene blue (EMB) agar (HiMedia laboratories, Mumbai, India) and incubated at 35˚C with and without 5% CO2 for 24-48 hours to ensure the absence of any microorganisms in the chocolate.
Preparation of Probiotic Chocolate
Preparation of the probiotic chocolate was carried according to the WHO/FDA guidelines which stated that probiotic bacteria should be present at a concentration of 108 CFU/g in probiotic food substances. 100 mL of probiotic bacteria inoculum with 108 CFU/mL (matched with McFarland 0.5) was added to 100 g of 68% dark chocolate (chocolate without any milk or nutrition bar, containing cocoa, cocoa butter, carbohydrate 64.6%).
Antibacterial activity of the probiotic chocolate by agar well diffusion technique
The efficacy of the probiotic chocolate combinations and probiotic combinations without chocolate was assessed by agar well diffusion technique and the diameters of the zone of inhibition (in mm) around the wells were recorded. Wells of eight mm diameter were punched out on BHIA plates and seeded with S. mutans MTCC497 using sterile cork borers. The probiotic combinations and probiotic chocolate combinations were added to the respectively labeled wells in BHIA medium and incubated at 35˚ C in 5% CO2 for 24 hours. After overnight incubation, the diameters of the zone of inhibition around the wells were measured and recorded in millimeters (mm) (Fig 1). The antibacterial efficacies of the probiotic formulations (with and without chocolate) were compared to evaluate whether the chocolate could / could not alter the efficacy of the formulations. Sterile plain chocolate was included as negative control. The assay was performed in triplicates.
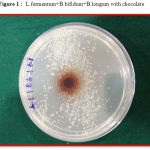 |
Figure 1: L.fermentum+B.bifidum+B.longum with chocolate |
The seven groups were divided into two subgroups based on the presence or absence of chocolate (Table 1)
Table 1: Experimental groups
| GROUPS | |
| I | L.fermentum |
| II | L.delbrueckii |
| III | B.bifidum |
| IV | B.longum |
| V | L.fermentum+L.delbrueckii |
| VI | B.bifidum+B.longum |
| VII | L.fermentum+B.bifidum+B.longum |
Results
The zones of inhibition obtained for all probiotic strains with, and without chocolate against Streptococcus mutans were calculated and tabulated. Between the species under study, the highest inhibitory effect was depicted by Bifidobacterium Longum when used alone and with probiotic chocolate. The combination of Lactobacillus fermentum, Bifidobacterium longum and Bifidobacterium bifidum proved to be better than all the probiotics in the study either in presence or absence of chocolate. In the present study, the least effect was shown by the probiotic chocolate with only Lactobacillus delbrueckii.(Table 2)
Table 2: diameter (mm) of the zone of inhibition
| Probiotic formulation |
Probiotic strain | Diameter (mm) of the Zone of inhibition | |||||
| With Chocolate | Without Chocolate | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| I | L.fermentum | 12 | 11 | 13 | 13 | 12 | 13 |
| II | L.delbrueckii | 10 | 10 | 11 | 11 | 11 | 10 |
| III | B.bifidum | 14 | 13 | 13 | 12 | 13 | 12 |
| IV | B.longum | 13 | 14 | 14 | 13 | 13 | 14 |
| V | L.fermentum+L.delbrueckii | 13 | 12 | 13 | 15 | 14 | 14 |
| VI | B.bifidum+B.longum | 15 | 14 | 15 | 13 | 12 | 12 |
| VII | L.fermentum+B.bifidum+B.longum | 18 | 18 | 16 | 15 | 15 | 16 |
Statistical Analysis
The values were statistically analysed using ONE-WAY ANOVA. The results showed that there was statistical significant difference between the probiotic strains [P<0.001].(Table 3)
Table 3: Statistical analysis
| Probiotic strain | S.mutans count | |||
| With Chocolate | Without Chocolate | |||
| Mean | SD | Mean | SD | |
| L.fermentum | 12.00 | 1.00 | 12.67 | .58 |
| L.delbrueckii | 10.33 | .58 | 10.67 | .58 |
| B.bifidum | 13.33 | .58 | 12.33 | .58 |
| B.longum | 13.67 | .58 | 13.33 | .58 |
| L.fermentum+L.delbrueckii | 12.67 | .58 | 14.33 | .58 |
| B.bifidum+B.longum | 14.67 | .58 | 12.33 | .58 |
| L.fermentum+B.bifidum+
B.longum |
17.33 | 1.15 | 15.33 | .58 |
| P value | <0.001** | ˂0.001** | ||
Discussion
Dental caries can be prevented by various methods. Recently, probiotics has gained importance in the prevention of dental caries. Probiotics are incorporated in foods and beverages, especially fruit juices, and fermented milk products like curd, yoghourt drinks, cheese, lassi, ice-cream, kulfi, whey beverages and probiotic in the form of powder, capsules and gelatin tablets, which are currently prescribed by physicians in conjunction with antibiotic therapy8.
In this study, Chocolate was selected as a vehicle for Lactobacillus and Bifidobacterium6. Instead of plain candies containing only sugar, which decompose and create feasible environment for growth of S. mutans, dark chocolate was used due to the presence of flavonoids, which possess various therapeutic properties and a prebiotic effect enhancing the antibacterial activity of probiotic chocolate.
Secretion of anti-microbial substances by probiotics such as bacteriocins, bacteriocin like substances (BLS) inhibits cell adhesion, colonization and invasion of pathogenic bacteria, especially the cariogenic bacteria9, 10. Bacteriocins inhibit pathogenic organisms by acting as colonizing peptides, which facilitates dominance of producer in a particular niche 11; act as killing peptide, which inhibits competing strains or pathogens directly 12; act as signaling peptide which either signals other bacteria through quorum sensing on signaling cells of the immune system of the host 13. S.mutans is found to produce a bacteriocin called mutacin 1140 14. Therefore, probiotic strain which will have an antagonist effect to the mutacin produced by s.mutans will have a better anti-cariogenic property. Some of the bacteriocins produced by Bifidobacterium are bifidiocin I, bifidiocin B and bacteriocin like inhibitory substances 15. Similarily Lactobacillus produces lactacin F16.
In the oral cavity, the mechanisms of action of probiotic containing dietary products when consumed are hypothesized as follows:
Direct interaction in dental plaque which interferes with the formation of biofilm, disrupts plaque ecology and resulting in competitive inhibition of the oral microbes along with production of antimicrobial substances.
Indirect action includes modulation of systemic immunity, local immunity, non-immunologic defense mechanisms, altered mucosal permeability and colonization by less pathogenic species17.
The enhancement of probiotic activity by the organism under study may be due to the various pre-biotic constituents in cocoa such as inulin and flavonoids, which inhibit the growth of the potent and prevalent cariogenic organisms, S.mutans. Polyphenols like flavonoids and proanthocyanidins from plant stimulant beverages like cocoa, coffee and tea were found to reduce biofilm formation and acid production by S.mutans 4. Cocoa phenolics are bioactive compounds possessing anti-oxidant, anti-radical, antiplatelet, anti-inflammatory, anti-carcinogenic and anti-cariogenic products and are also considered to be prebiotics18.
Bifidobacterium was found to produce more amounts of bacteriocins whereas only certain species of Lactobacillus were found to produce bacteriocins. The bacteriocins produced by the lactobacillus are considered minimal when compared to Bifidobacteria 19. This justifies wider zone of inhibition in the Bifidobacteria group when compared to the lactobacillus group. The results of our study showed that the combination group containing L. fermentum, B. bifidum and B. longum was found to have the highest inhibitory effect on S. mutans. This can be justified by the highest production of bacteriocins when the organisms were used in combination than singly.
Although probiotics are found to inhibit the pathogenic microorganisms by production of bacteriocins, the exact mechanism of inhibition of S. mutans by the probiotic organisms are yet to be evaluated.
The results of our study showed that there is a definite inhibitory effect of probiotic bacteria on S.mutans, which is significantly enhanced by the presence of chocolate, thus the null hypothesis is rejected.
Further studies should be carried out to evaluate the side effects of prescribing probiotic chocolates. Randomized controlled trials should be conducted before making recommendation of probiotic chocolate. The shelf life of probiotic chocolate was found to be lesser than the normal chocolate, and the storage conditions should be strictly followed in order to maintain the viability of the probiotic bacteria 20.
Within the limitations of this study, it can be concluded that probiotic chocolates containing Lactobacillus fermentum, Lactobacillus delbrueckii, Bifidobacterium bifidum and Bifidobacterium longum are found to significantly inhibit the growth of Streptococcus mutans. Dark chocolate with probiotic combination of Lactobacillus fermentum, Bifidobacterium bifidum and Bifidobacterium longum was found to have the highest inhibitory effect on Streptococcus mutans. Further extensive clinical studies are needed to augment the above observations and findings of the role of probiotic chocolate in inhibition and prevention of dental caries.
References
- Loesche, W. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986;50: 353-380.
- Geddes, D. A. M. Acids produced by human dental plaque metabolism in situ. Caries Res 1975; 9: 98-109.
- W.H. Bowen, H. Koo. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011; 45: 69–86
- Stamatova I. Probiotic activity of Lactobacillus delbrueckii subsp. bulgaricus in the oral cavity. Helsinky: Helsinky; 2010.
- Kligler B, Cohrssen A. Probiotics. Am Fam Physician. 2008;78 (9):1073-8
- Caglar E, Kargul B, Tanboga I. Bacteriotherapy and probiotics’ role on oral health. Oral Dis. 2005;11 (3):131-7.
- Khanafari A., Hosseini Porgham S. and Tajabadi Ebrahimi M. An investigation of probiotic chocolate effect on Streptococcus mutans growth inhibition. Jundishapur Journal of Microbiology 2012; 5(4):498-505
- Schwarzer, M.; Srutkova, D.; Schabussova, I.; Hudcovic, T.; Akgün, J.; Wiedermann, U.; Kozakova, H. Neonatal colonization of germ-free mice with Bifidobacterium longum prevents allergic sensitization to major birch pollen allergen Bet v 1. Vaccine 2013, 31, 5405–5412.
- Bhushan J, Chachra S. Probiotics-their role in prevention of dental caries. J Oral Health Comm Dent. 2010;4 (3):78-82.
- Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, Cresci A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr. 2009;48 (6):355-63
- Riley MA, Wertz JE. Bacteriocin diversity: ecological and evolutionary perspectives. Biochem 2002. 84:357–364.
- Majeed H, Gillor O, Kerr B, Riley MA.Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J. 2011;5:71– 81.
- Czárán TL, Hoekstra RF, Pagie L. Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. U. S. A. 2002; 99:786–790.
- Hillman JD, Dzuback AL, Andrews SW.. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res.1987; 66:1092–1094
- Shadan Abas. Al- Wendawi, Adil Abead. Al- Saady. Screening for Bacteriocins Production in Enteric Bifidobacterium Isolates and Study of Some Production Affecting Factors. Medical Journal of Babylon 2012; 9:386-96.
- José Luis Parada, Carolina Ricoy Caron, Adriane Bianchi P. Medeiros and Carlos Ricardo Soccol Bacteriocins from Lactic Acid Bacteria: Purification, Properties and use as Biopreservatives . Brazilian journal of biology and technology 2007;50:521-542
- Meurman JH. Probiotics: Do they have a role in oral medicine and dentistry? Eur J Oral Sci 2005;113:188‑96
- Redovniković IR , Delonga K, Mazor S, Dragović-Uzelac V, Carić M, Vorkapić-Furač J . Polyphenolic content and composition, and anti-oxidative activity of different cocoa liquors. Czech J Food Sci. 2009; 27: 330–7.
- Hoda Mahrous, Abeer Mohamed, M. Abd El-Mongy, A. I. El-Batal, H. A. Hamza Study Bacteriocin Production and Optimization Using New Isolates of Lactobacillus Isolated from some Dairy Products under Different Culture Conditions. Food and Nutrition Sciences, 2013; 4: 342-356.
- Christopher M, Reddy VP, Venkateswarlu K. Viability during storage of two Bifidobacterium bifidum strains in set and stirred flavoured yoghurts containing whey protein concentrates. Nat Prod Rad. 2009;8:25-31.








